Abstract
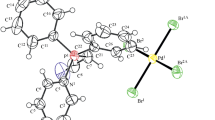
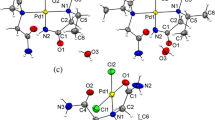
The effect of the nature of halogen-substituted carboxylic acids RCOOH, where R is ClCH2, Cl2CH, Cl3C, or F3C, on the complexation of palladium halocarboxylates with morpholine C4H9NO was investigated. Reactions with ClCH2COOH and Cl2CHCOOH gave binuclear complexes [(C4H9NO)2Pd2(μ-OOCR)2(OOCR)2] with palladium-coordinated morpholine, whereas reactions with Cl3CCOOH and F3CCOOH afforded the first tetra(halocarboxylate) palladium complexes with protonated morpholine as the cation, (C4H10NO)2[Pd(RCOO)4]. The acid–base balance of morpholine and halocarboxylic acid was the key factor determining the composition of the resulting complexes. For the formation of palladium tetra(halocarboxylates) with morpholine, the difference between the morpholine and acid pKa values should be not lower than 7.63. X-ray diffraction studies were carried out for the first tetra(halocarboxylate) palladium complex with a monocarboxylic acid (C4H10NO)2[Pd(OOCCF3)4 ∙ 2H2O] (I) and for trans-[(C4H9NO)2Pd(OOCCH2Cl)2 ∙ 2H2O] (II), trans-[(C4H9NO)2Pd(OOCCHCl2)2] (III), and trans-[(C4H9NO)2Pd(OOCCF3)2 ∙ 2H2O] (IV) (CIF files CCDC nos. 1008564, 1894300, 1008566, and 1894299, respectively).






Similar content being viewed by others
REFERENCES
Rosenberg, B., Plat. Met. Rev., 1971, vol. 15, no. 2, p. 42.
Lippert, B., Cisplatin: Chemistry and Biochemistry of Leading Anticancer Drug, Weinheim: Wiley−VCH, 1995.
Fulford, A., Plat. Met. Rev., 1966, vol. 40, no. 4, p. 161.
Giachetti, S., Perpoint, B., Zidani, R., et al., J. Clin. Oncol., 2000, vol. 18, p. 136.
Weiss, R.V. and Poster, D.S., Cancer Treat. Rev., 1982, vol. 9, p. 37.
Groth, S., Nielsen, H., Sorensen, J., et al., Cancer Chemother. Pharm., 1986, vol. 17, p. 191.
Petrov, V.I., Fisenko, V.P., Arzamatsev, E.V., et al., Guide on Experimental (Pre-Clinical) Study of New Substances for Pharmacology, Moscow, 2000, p. 398.
Brienza, S., Vignoud, J., Itznaki, M., et al., Proc. Am. Soc. Clin. Oncol., 1995, vol. 14, p. 209.
Dress, M., Dengler, W.M., Hendrics, H.R., et al., Eur. J. Cancer. A, 1995, vol. 31, p. 356.
Levi, F., Perpoint, B., Garuit, C., et al., Eur. J. Cancer. A, 1993, vol. 29, p. 1608.
Tusekbozic, L., Furlani, A., Scarcia, V., and Balzarini, E., J. Inorg. Biochem., 1998, vol. 72, nos. 3–4, p. 201.
Wimmer, F.L., Wimmer, S., Castan, P., Cros, S., et al., Anticancer Res., 1989, vol. 9, p. 791.
Fiallo, M.M. and Garnier-Suillerot, A., Inorg. Chem., 1987, vol. 137, p. 119.
Quroga, A.G., Perez, J.M., Montero, E.J., et al., J. Inorg. Biochem., 1999, vol. 75, no. 4, p. 293.
Ivanova, N.A., Kurbakova, A.P., Erofeev, V.V., et al., Zh. Neorg. Khim., 1991, vol. 36, no. 11, p. 2821.
Bouquillon, S., D’Hardemare, A.M., Averbuch-Pouchot, M.T., et al., Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1999, vol. 55, p. 2028.
Pointtillart, F., Train, C., Villain, F., et al., J. Am. Chem. Soc., 2007, vol. 129, p. 1327.
Ammar, R.A.A., Fluide Phase Equilibria, 2009, vol. 285, p. 116.
Plutin, A.M., Mocelo, R., Alvarez, A., et al., J. Inorg. Biochem., 2014, vol. 134, p. 76.
Barrac, V., Roch, F.V., Morel, L., et al., Inorg. Chim. Acta, 2016, vol. 446, p. 54.
Zakharova, I.A. (Efimenko), Issledovaniya po neorganicheskoi khimii i khimicheskoi tekhnologii (Studies in Inorganic Chemistry and Chemical Engineering), Moscow: Nauka, 1988, p. 171.
Efimenko, I.A. and Shishilov, O.N., Russ. J. Inorg. Chem., 2012, vol. 57, no. 14, p. 1695.
Efimenko, I.A., Churakov, A.V., Ivanova, N.A., et al., Russ. J. Inorg. Chem., 2017, vol. 62, no. 11, p. 1469.
Grekhova, A.K., Gorbacheva, L.B., Ivanova, N.A., and Efimenko, I.A., Zh. Biomed. Khim., 2013, vol. 59, no. 1, p. 107.
Efimenko, I.A., Shishilov, O.N., Ivanova, N.A., and Erofeeva, O.S., Precious Met., 2012, vol. 33, p. 240.
Cherkashina, N.V., Kozitcyna, N.Yu., Aleksandrova, G.G., et al., Mend. Commun., 2002, vol. 12, no. 2, p. 49.
Efimenko, I.A., Ankudinova, P.V., Kuz’mina, L.G., et al., Russ. J. Inorg. Chem., 2015, vol. 60, no. 7, p. 848.
Shishilov, O.N., Stromnova, T.A., Churakov, A.V., et al., Russ. J. Inorg. Chem., 2006, vol. 51, no. 4, p. 574.
Sheldrick, G.M., SADABS. Program for Scaling and Correction of Area Detector Data, Göttingen: Univ. of Göttingen, 1997.
Sheldrick, G.M., Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, vol. 64, no. 1, p. 31.
Allen, F.H., Acta. Crystallogr., Sect. B: Sctruct. Sci., 2002, vol. 58, no. 1, p. 380.
Efimenko, I.A., Podobedov, R.T., Churakov, A.V., et al., Russ. J. Coord. Chem., 2011, vol. 37, no. 8, p. 625. https://doi.org/10.1134/S1070328411080021
Pandey, R.N. and Henry, P.M., Can. J. Chem., 1974, vol. 52, p. 1241.
Kukushkin, Yu.N., Khimiya koordinatsionnykh soedinenii (Chemistry of Coordination Compounds), Moscow: Vysshaya Shkola , 1955.
Nakamoto, K., Infrared Spectra and Raman Spectra of Inorganic and Coordination Compounds, New York: Wiley, 1986.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by Z. Svitanko
Rights and permissions
About this article
Cite this article
Efimenko, I.A., Churakov, A.V., Erofeeva, O.S. et al. Effect of the Nature of Haloacetic Acids on the Type of Morpholine Complexes Formed. Crystal Structure of the First Palladium Tetracarboxylate with Monocarboxylic Acid: Morpholinium Tetrakis(trifluoroacetato)palladate(II), (O(CH2CH2)2NH2)2[Pd(CF3COO)4]. Russ J Coord Chem 45, 615–625 (2019). https://doi.org/10.1134/S1070328419090033
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070328419090033