Abstract
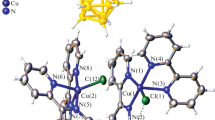
The structure of the complex [Cu(Bipy)2(BA)] ∙ 7H2O (I), where Bipy is 2,2'-dipyridyl, and BA2– is the barbituric acid anion (H2BA), is determined (CIF file CCDC no. 1887338). The thermal decomposition and IR spectrum of complex I are studied. The crystals are orthorhombic: a = 26.118(3), b = 27.685(3), c = 15.683(2) Å, V = 11 370(2) Å3, space group Fdd2, Z = 16. The discrete structure of the polar crystal consists of neutral [Cu(Bipy)2(BA)] particles and molecules of crystallisation water . The Cu2+ ion is bound to the N atoms of two bidentate Bipy molecules and the N atom of the BA2− ion at the vertices of the trigonal bipyramid CuN5. Compound I is the first example of the metal complex only with the N-coordinated anions of barbituric acid (BA2−, НBA−). The structure is stabilized by hydrogen bonds O−H∙∙∙O and N−H∙∙∙O to form a three-dimensional network with the π–π interaction between the Bipy molecules. The compound begins to lose water at ~50°С and is completely dehydrated above 200°С.



Similar content being viewed by others
REFERENCES
Mahmudov, K.T., Kopylovich, M.N., Maharramov, A.M., et al., Coord. Chem. Rev., 2014, vol. 265, p. 1.
Xiong, Y., He, C., An, N.C., et al., Transition Met. Chem., 2003, vol. 28, no. 1, p. 69.
Gryl, M., Acta Crystallogr., Sect. B: Struct. Sci., Cryst. Eng. Mater., 2015, vol. 71, no. 4, p. 392.
Chu, J., Liu, Z.Y., Zhao, X.J., and Yang, E.C., Russ. J. Coord. Chem., 2010, vol. 36, no. 12, p. 901. https://doi.org/10.1134/S1070328410120067
Golovnev, N.N., Solovyov, L.A., Lesnikov, M.K., et al., Inorg. Chim. Acta, 2017, vol. 467, p. 39.
Braga, D., Grepioni, F., Lampronti, G.I., et al., Cryst. Growth Des., 2011, vol. 11, no. 12, p. 5621.
Braga, D., Grepioni, F., Maini, L., et al., CrystEngComm, 2012, vol. 14, p. 3521.
Solovyov, L.A., Golovnev, N.N., Molokeev, M.S., and Lesnikov, M.K., J. Coord. Chem., 2017, vol. 70, p. 1884.
Golovnev, N.N., Molokeev, M.S., Lesnikov, M.K., et al., Russ. J. Inorg. Chem., 2017, vol. 62, p. 746. https://doi.org/10.1134/S0036023617060092
Chierotti, M.R., Gaglioti, K., Gobetto, R., et al., Cryst-EngComm, 2013, vol. 15, p. 7598.
Gryl, M. and Stadnicka, K., Acta Crystallogr., Sect. E: Struct. Rep. Online, 2011, vol. 67, p. m571.
Braga, D., Grepioni, F., Maini, L., Prosperi, S., et al., Chem. Commun., 2010, vol. 46, p. 7715.
Golovnev, N.N., Molokeev, M.S., Sterkhova, I.V., and Lesnikov, M.K., Inorg. Chem. Commun., 2018, vol. 97, p. 88.
Sinn, E., Flynn, C.M., Jr., and Martin, R.B., J. Am. Chem. Soc., 1978, vol. 100, no. 2, p. 489.
Türkel, N. and Aksoy, M.S., ISRN Anal. Chem., 2014, vol. 2014, p. 1.
Korpi, H., Sippola, V., Filpponen, I., et al., Appl. Catal., A, 2006, vol. 302, no. 2, p. 250.
Garcia, H.C., J. Coord. Chem., 2011, vol. 64, p. 1125.
Gerasimova, T.P. and Katsyuba, S.A., Dalton Trans., 2013, vol. 42, p. 1787.
Sheldrick, G.M., Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, vol. 64, no. 1, p. 112.
PLATON. A Multipurpose Crystallographic Tool, Utrecht (Netherlands): Univ. of Utrecht, 2008.
Brandenburg, K. and Berndt, M., DIAMOND, Visual Crystal Structure Information System CRYSTAL IMPACT, Postfach, 2005, p. 1251.
Cambridge Structural Database. Version 5.36, Cambridge: Univ. of Cambridge, 2018.
Lewis, T.C., Tocher, D.A., and Price, S.L., Cryst. Growth Des., 2004, vol. 4, no. 5, p. 979.
Golovnev, N.N. and Molokeev, M.S., 2-Tiobarbiturovaya kislota i ee kompleksy s metallami: sintez, struktura i svoistva (2-Thiobarbituric Acid and Its Metal Complexes: Synthesis, Structure, and Properties), Krasnoyarsk: Sib. Feder. Univ., 2014.
Steed, J.W. and Atwood, J.L., Supramolecular Chemistry, Moscow: Akademkniga, 2007, Ch. 1−2.
ACKNOWLEDGMENTS
The X-ray diffraction data were obtained using the equipment of the Baikal and Krasnoyarsk Centers for Collective Use (Siberian Branch of the Russian Academy of Sciences).
Funding
This work was supported by the Ministry of Education and Science of the Russian Federation in the framework of the state task for the Siberian Federal University for 2017–2019 (4.7666.2017/BCh).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by E. Yablonskaya
Rights and permissions
About this article
Cite this article
Golovnev, N.N., Molokeev, M.S., Sterkhova, I.V. et al. Structure of Barbituratobis(2,2'-Dipyridyl)copper(II) Heptahydrate. Russ J Coord Chem 45, 569–572 (2019). https://doi.org/10.1134/S1070328419080037
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070328419080037