Abstract
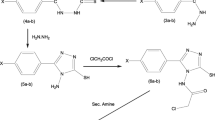
Some new 1-substituted-4-(4-nitrophenyl)-[1,2,4]triazolo[4,3-a]quinazolin-5(4H)-ones were synthesized and screened for their H1-antihistaminic activity. The structures of the newly synthesized compounds were confirmed by IR, 1H NMR, and mass spectral data and purity of the compounds was determined by elemental analysis. Antihistaminic activity of synthesized compounds was determined by the protection against histamine-induced bronchospasm on conscious guinea pigs. Percentage protection data showed that all title compounds of the series show significant protection in the range of 68–71.56% when compared to reference drug chlorpheniramine maleate (70.17%). The sedative properties of the compounds were also evaluated and found to be negligible when compared to chlorpheniramine maleate.
Similar content being viewed by others
REFERENCES
Carr, A.A. and Meyer, D.R., Arzneimittelforschung, 1982, vol. 32, pp. 1157–1159.
E.F. Ellis, N.F. Adkinson, J.W. Yungingen, and W.W. Busso, H1-receptor antagonists, in Musby-Year Book, Inc., 1985, pp. 856–891.
Mallareddy, V. and Sattur, P.B., Curr. Sci., 1984, vol. 53, pp. 1069–1075.
Kumar, S., Shrivastava, S.K., and Sarkar, P.C., Indian J. Heterocycl. Chem., 1997, vol. 7, 109–113.
Bartroli, J., Turmo, E., and Alguero, M., J. Med. Chem., 1988, vol. 41, pp. 1869–1892.
Alagarsamy, V., Sharma, H.K., Parthiban, P., Singh, J.C., Murugan, S.T., and Solomon, V.R. Pharmazie, 2009, vol. 64, pp. 5–12.
Bousquet J., Van Cauwenberge, P., and Khaltaev, N., J. Allergy Clin. Immunol., 2001, vol. 108, pp. 147–152.
Kuhn, W.L. and Van Maanen, E.F., J. Pharmacol. Exp. Ther., 1961, vol. 134, pp. 60–68.
Van Arman, C.G., Miller, L.M., and O’Malley, M.P., J. Pharmacol. Exp. Ther., 1961, vol. 133, pp. 90–97.
Alagarsamy, V., Narendhar, B., Sulthana, M.T., and Solomon, V.R., Med. Chem. Res., 2014, vol. 23, pp. 4692–4299.
Nivedhitha, S., Solomon, V.R., Gobinath, M., Subramanian N., and Alagarsamy, V., Trop. J. Pharm. Res., 2015, vol. 14, pp. 271–278.
Mallareddy, V., Ravinderreddy, P., and Jayamma, Y., Indian Drugs, 1987, vol. 25, pp. 182–186.
Raghuram Rao, A., Chandrasekhar, V., and Mallareddy, V., Indian Drugs, 1986, vol. 24, pp. 40–48.
Hopp, R.J., Bewtra, A., Nair, N.M., and Townley, R.G., J. Allergy Clin. Immunol., 1985, vol. 76, pp. 609–614.
Raju, V.S.K., Bhagawanraju, M., Bahekar, R.H., Rajan, K.S., and Raghuram Rao, A. Indian Drugs, 1999, vol. 36, pp. 759–764.
Chairungsrilerd, N., Furukawa, K., Ohta, T., Nozoe, S., and Ohizumi, Y., Eur. J. Pharmacol., 1996, vol. 314, pp. 351–359.
Dews, P. B., Br. J. Pharmacol., 1953, vol. 8, pp. 46–52.
Kuhn, W.L. and Van Maanen, E.F., J. Pharmacol. Exp. Ther., 1961, 134, pp. 60–67.
Mukherji, D.D., Nintiyal, S.R., Prasad, C.R., and Dhawan, B.N. Indian J. Med. Res., 1980, vol. 93, pp. 1426–1467.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
COMPLIANCE WITH ETHICAL STANDARDS
The Institutional Animal Ethics committee approved the protocol adopted for the experimentation of animals. All applicable international, national, and institutional guidelines for the care and use of animals were followed.
Conflict of Interests
The authors report no conflicts of interest.
Additional information
Corresponding author: phone: +91 8455-230690; fax: +91 8455 233888; e-mail: drvalagarsamy@gmail.com.
Rights and permissions
About this article
Cite this article
Gobinath, M., Subramanian, N., Alagarsamy, V. et al. Design and Synthesis of 1-Substituted-4-(4-Nitrophenyl)-[1,2,4]triazolo[4,3-a]quinazolin-5(4H)-ones as a New Class of Antihistaminic Agents. Russ J Bioorg Chem 46, 403–408 (2020). https://doi.org/10.1134/S1068162020030085
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1068162020030085