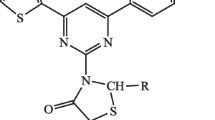
Abstract
3-(2-Thienyl)-5-aryl-1-thiocarbamoyl-2-pyrazolines were reacted with chloroacetone derivatives and hydrazonyl chloride derivatives in ethanol to afford the corresponding thiazolylpyrazoline derivatives and thiophenylpyrazolyl-5-substituted aryl-diazenylthiazole derivatives, respectively. The structures of the newly synthesized compounds were elucidated by different elemental and spectral analyses (IR, mass, 1H and 13C NMR). The antimicrobial and antifungal activities of the newly synthesized compounds were evaluated against four bacterial species and five fungal strains. In addition, the antitumor activities of two of the newly synthesized compounds 1-(2-(5-(4-chlorophenyl)-3-(thiophen-2-yl)-4,5-dihydropyrazol-1-yl)-4-methyl thiazol-5-yl)ethan-1-one and 2-(5-(4-chlorophenyl)-3-(thiophen-2-yl)-4,5-dihydro-1H-pyrazol-1-yl)-4-methyl-5-(phenyl-diazenyl)thiazole against HEPG-2, HCT-116, MCF-7, BHK, and CACO-2 were evaluated. From the obtained results, we found that these two compounds were the most potent candidates towards all gram-positive and gram-negative bacteria, as well as the fungi studied. Also, the same two compounds showed strong antitumor activities against two of the tumor cell lines (HCT-116 and CACO-2).
Similar content being viewed by others
REFERENCES
Fustero, S., Sánchez-Roselló, M., Barrio, P., and Simón-Fuentes, A., Chem. Rev., 2011, vol. 111, pp. 6984–7034. https://doi.org/10.1021/cr2000459
Ansari, A., Ali, A. and Asif, M., New J. Chem., 2017, vol. 41, pp. 16–41. https://doi.org/10.1039/C6NJ03181A
Kumar, S., Bawa, S., Drabu, S., Kumar, R., and Gupta, H., Recent Pat. Anti-Infect. Drug Discov., 2009, vol. 4, pp. 154–163.
Shaaban, M.R., Mayhoub, A.S., and Farag, A.M., Expert Opin. Ther. Pat., 2012, vol. 22, pp. 253–291.
Marella, A., Ali, R., Alam, T., Saha, R., Tanwar, O., Akhter, M., Shaquiquzzaman, M., and Alam, M.M., Mini-Rev. Med. Chem., 2013, vol. 13, pp. 921–931.
Alex, J.M. and Kumar, R., J. Enzyme Inhib. Med. Chem., 2014, vol. 29, pp. 427–442.
Havrylyuk, D., Zimenkovsky, B., Vasylenko, O., Zaprutko, L., Gzella, A., and Lesyk, R., Eur. J. Med. Chem., 2009, vol. 44, pp. 1396–1404.
Shaharyar, M., Abdullah, M.M., Bakht, M.A., and Majeed, J., Eur. J. Med. Chem., 2010, vol. 45, pp. 114–119.
Congiu, C., Onnis, V., Vesci, L., Castorina, M., and Pisano, C., Bioorg. Med. Chem., 2010, vol. 18, pp. 6238–6248.
Al-Abdullah, E.S., Molecules, 2011, vol. 16, p. 3410–3419.
Bano, S., Javed, K., Ahmad, S., Rathish, I.G., Singh, S., and Alam, M.S., Eur. J. Med. Chem., 2011, vol. 46, pp. 5763–5768.
Bashir, R., Ovais, S., Yaseen, S., Hamid, H., Alam, M.S., Samim, M., Singh, S., and Javed, K., Bioorg. Med. Chem. Lett., 2011, vol. 21, pp. 4301–4305.
Amin, K.M., Eissa, A.A.M., Abou-Seri, S.M., Awadallah, F.M., and Hassan, G.S., Eur. J. Med. Chem., 2013, vol. 60, pp. 187–198.
Montoya, A., Quiroga, J., Abonia, R., Nogueras, M., Cobo, J., and Insuasty, B., Molecules, 2014, vol. 19, pp. 18 656–18 675. https://doi.org/10.3390/molecules191118656
Insuasty, B., Montoya, A., Becerra, D., Quiroga, J., Abonia, R., Robledo, S., Darío Vélez, I., Upegui, Y., Nogueras, M., and Cobo, J., Eur. J. Med. Chem., 2013, vol. 67, pp. 252–262.
Rathore, P., Yaseen, S., Ovais, S., Bashir, R., Yaseen, R., and Hameed, A.D., Samim, M., Gupta, R., Hussain, F., and Javed, K., Bioorg. Med. Chem. Lett., 2014, vol. 24, pp. 1685–1691.
Lv, P.-C., Li, D.-D., Li, Q.-S., Lu, X., Xiao, Z.-P., and Zhu, H.-L., Bioorg. Med. Chem. Lett., 2011, vol. 21, pp. 5374–5377.
Shin, S.Y., Yoon, H., Hwang, D.; Ahn, S., Kim, D.-W., Koh, D., Lee, Y.H., and Lim, Y., Bioorg. Med. Chem., 2013, vol. 21, pp. 7018–7024.
Yu, M., Yang, H., Wu, K., Ji, Y., Ju, L., and Lu, X., Bioorg. Med. Chem., 2014, vol. 22, pp. 4109–4118.
Amin, K.M., Abou-Seri, S.M., Awadallah, F.M., Eissa, A.A.M., Hassan, G.S., and Abdulla, M.M., Eur. J. Med. Chem., 2015, vol. 90, pp. 221–231.
Qin, Y.-J., Li, Y.-J., Jiang, A.-Q., Yang, M.-R., Zhu, Q.-Z., Dong, H., and Zhu, H.-L., Eur. J. Med. Chem., 2015, vol. 94, pp. 447–457.
Mishra, C.B., Kumari, S., and Tiwari, M., Eur. J. Med. Chem., 2015, vol. 92, pp. 1–34.
Ayati, A., Emami, S., Asadipour, A., Shafiee, A., and Foroumadi, A., Eur. J. Med. Chem., 2015, vol. 97, pp. 699–718.
Sun, Z.Q., Tu, L.X., Zhuo, F.J., and Liu, S.X., Bioorg. Med. Chem. Lett., 2016, vol. 26, pp. 747–750.
D’Ascenzio, M., Chimenti, P., Gidaro, M.C., De Monte, C., De Vita, D., Granese, A., Scipione, L., Di Santo, R., Costa, G., Alcaro, S., Yáñez, M., and Carradori, S., J. Enzyme Inhib. Med. Chem., 2015, vol. 30, pp. 908–919.
Zala, A.V., Walker, M.M., and Talley N.J., Expert Opin. Emerg. Drugs, 2015, vol. 20, pp. 221–233.
Logu, A.D., Sadd, M.I., Cardia, M.C., Borgna, R., Sanna, C., Saddi, B., and Elias, M., J. Antimicrob.Chemother., 2005, vol. 55, pp. 692–698.
Liu, C.L., Li, Z.M., and Zhong, B., J. Fluorine Chem., 2004, vol. 125, pp. 1287–1290.
Kumar, R.V.K. and Kumar, V.S.R.S., J. Heterocycl. Chem., 2005, vol. 42, pp. 1191–1193.
D’Andrea, S., Zheng, Z.B., DenBleyker, K., Fung-Tomc, J.C., Yang, H., Clark, J., Taylor, D., and Bronson, J., Bioorg. Med. Chem. Lett., 2005, vol. 15, pp. 2834–2839.
Azarifar, D. and Shaebanzadeh, M., Molecules, 2002, vol. 7, pp. 885–895.
Matysiak, J. and Niewiadom A.Y., Bioorg. Med. Chem., 2003, vol. 11, pp. 2285–2287.
Jungheim, L.N., Sigmund, S.K., and Fisher, J.W., Tetrahedron Lett., 1987, vol. 28, pp. 285–288.
Temel, H.E., Altintop, M.D., and Özdemir, A., Turk. J. Pharm. Sci., 2018, vol. 15, pp. 333–338. https://doi.org/10.4274/tjps.20982
Nassar, I.F., Atta-Allah, S.R., and Elgazwy, A-S.S.H., J. Enz. Inhib. Med. Chem., 2015, vol. 30, pp. 396–405. https://doi.org/10.3109/14756366.2014.940936
Nassar, I.F., El Farargy, A.F., Abdelrazek, F.M., and Ismail, N.S.M., Nucleosides, Nucleotides Nucleic Acids, 2017, vol. 36, pp. 275–291. https://doi.org/10.1080/15257770.2016.1276290
Nassar, I.F., El Farargy, A.F., and Abdelrazek, F.M., J. Heterocycl. Chem., 2018, vol. 55, pp. 1709–1718. https://doi.org/10.1002/jhet.3208
Nassar, I.F., Atta-Allah, S.R., and Hemdan M.M., Phosphorus Sulphur, 2018, vol. 193, pp. 630–636. https://doi.org/10.1080/10426507.2018.1487435
Nassar, I.F., EL-Kady D.S., Awad H.M., and El-Sayed W.A., J. Heterocycl. Chem., 2019, vol. 56, pp. 1086–1100. https://doi.org/10.1002/jhet.3496
Kabli, R.A., Khalaf, A.A., Zimaity, M.T., Khalil, A.M., Kaddah, A.M., and Al-Rifaie, H.A., J. Indian Chem. Soc., 1991, vol. 68, pp. 47–51.
Wiley, R.H., Jarboe, C.H., Hayes, F.N., Hansbury, E., Nielsen, J.T., and Callahan, P.X., J. Org. Chem., 1958, Vol. 23, pp. 732–738.
Abdel-Kader, H.A. and Seddkey, S.R., Assiut Vet. Med. J., 1995, vol. 34, pp. 67.
Fagbemi, J., Ferdinand, L., and Adenipekun, T., Afr. J. Biotech., 2009, vol. 8, pp. 1176–1182.
Skehan, P. and Storeng, R., J. Natl. Cancer Inst., 1990, vol. 82, pp. 1107–1112.
Eweiss, N.F. and Osman, A., J. Heterocycl. Chem., 1980, vol. 17, pp. 1713–1717.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
COMPLIANCE WITH ETHICAL STANDARDS
The work has no studies involving humans or animals as subjects of the study.
Conflict of Interests
Authors declare they have no conflicts of interest.
Additional information
Corresponding author: e-mail: Dr.Ibrahim.Nassar@ sedu.asu.edu.eg.
Rights and permissions
About this article
Cite this article
Safaa I. Elewa, Mansour, E., Nassar, I.F. et al. Synthesis of Some New Pyrazoline-Based Thiazole Derivatives and Evaluation of Their Antimicrobial, Antifungal, and Anticancer Activities. Russ J Bioorg Chem 46, 382–392 (2020). https://doi.org/10.1134/S1068162020030061
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1068162020030061