Abstract
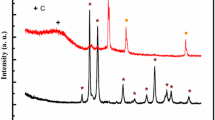
The nanocomposite samples, containing copper and iron species in the silica matrix, were prepared by annealing at temperatures up to 1100°C. The samples were investigated by X-ray diffraction analysis, Fourier transform infrared spectroscopy, and cyclic voltammetry. The results of the performed study depict to the presence of a temperature gradient, which acts on the sample during the annealing treatment in the furnace. For the first time, the influence of the temperature gradient on the formation mechanism of the samples was discussed.










Similar content being viewed by others
REFERENCES
C. J. Brinker and G. W. Scherer, Sol–Gel Science: The Physics and Chemistry of Sol–Gel Processing (Academic, Boston, 1990), p. 1.
V. N. Nikolic, Preparation and Characterization of Fe 2 O 3 -SiO 2 Nanocomposite for Biomedical Application, Mineralogy—Significance and Applications (IntechOpen, Rijeka, 2019).
O. M. Lemine, K. Omri, B. Zhang, L. El Mir, M. Sajieddine, A. Alyamani, and M. Bououdina, Superlatt. Microstruct. 52, 793 (2012).
Y. Sheng, J. Yang, F. Wang, L. Liu, H. Liu, C. Yan, and Z. Guo, Appl. Surf. Sci. 465, 154 (2019).
N. S. Kumar, R. P. Suvarna, and K. C. B. Naidu, Mater. Chem. Phys. 223, 241 (2019).
J. A. Lett, M. Sundareswari, K. Ravichandran, M. B. Latha, S. Sagadevan, and M. R. B. Johan, RSC Adv. 9, 6228 (2019).
T. Fardood, S. Ramazani, A. Moradnia, F. Afshari, Z. Ganjkhanlu, and F. Yekke Zare, Chem. Methodol. 3, 696 (2019).
K. Davis, R. Yarbrough, M. Froeschle, J. White, and H. Rathnayake, RSC Adv. 9, 14638 (2019).
R. Bao, C. Chen, J. Xia, H. Chen, and H. Li, J. Mater. Chem. C 7, 4981 (2019).
K. Suzuki, S. Sato, and M. Fujita, Nat. Chem. 2, 25 (2010).
F. I. H. Rhouma, F. Belkhiria, E. Bouzaiene, M. Daoudi, K. Taibi, J. Dhahri, and R. Chtourou, RSC Adv. 9, 5206 (2019).
D. S. Kolchanov, V. Slabov, K. Keller, E. Sergeeva, M. V. Zhukov, A. S. Drozdov, and A. V. Vinogradov, J. Mater. Chem. C 7, 6426 (2019).
A. S. Hassanien, A. A. Akl, and A. H. Saaedi, CrystEngComm 20, 1716 (2018).
M. V. Berezhnaya, O. V. Almyasheva, V. O. Mittova, A. T. Nguen, and I. Y. Mittova, Russ. J. Gen. Chem. 88, 626 (2018).
S. Nayak, A. Soam, J. Nanda, C. Mahender, M. Singh, D. Mohapatra, and R. Kumar, J. Mater. Sci.-Mater. Electron. 29, 9361 (2018).
U. Alam, A. Khan, D. Ali, D. Bahnemann, and M. Muneer, RSC Adv. 8, 17582 (2018).
W. F. Chen, S. S. Mofarah, D. A. H. Hanaor, P. Koshy, H. K. Chen, and Y. Jiang, Inorg. Chem. 57, 7279 (2018).
A. H. Ashour, A. I. El-Batal, M. A. Maksoud, G. S. El-Sayyad, S. Labib, E. Abdeltwab, and M. M. El-Okr, Particuology 40, 141 (2018).
K. Zheng and A. R. Boccaccini, Adv. Colloid Interface Sci. 249, 363 (2017).
T. Chen, Y. Ma, Q. Guo, M. Yang, and H. Xia, J. Mater. Chem. A 5, 3179 (2017).
S. T. Fardood, A. Ramazani, and S. W. Joo, J. Appl. Chem. Res. 11, 8 (2017).
Y. Sheng, J. Yang, F. Wang, L. Liu, H. Liu, C. Yan, and Z. Guo, Appl. Surf. Sci. 465, 154 (2019).
M. Salavati-Niasari, F. Soofivand, A. Sobhani-Nasab, M. Shakouri-Arani, M. Hamadanian, and S. Bagheri, J. Mater. Sci.: Mater. Electron. 28, 14965 (2017).
B. Ritter, P. Haida, T. Krahl, G. Scholz, and E. Kemnitz, J. Mater. Chem. C 5, 5444 (2017).
J. N. Hasnidawani, H. N. Azlina, H. Norita, N. N. Bonnia, S. Ratim, and E. S. Ali, Proc. Chem. 19, 211 (2016).
N. N. M. Zorkipli, N. H. M. Kaus, and A. A. Mohamad, Proc. Chem. 19, 626 (2016).
F. Wang, H. Li, Z. Yuan, Y. Sun, F. Chang, H. Deng, L. Xie, and H. Li, RSC Adv. 6, 79343 (2016).
T. M. Wandre, P. N. Gaikwad, A. S. Tapase, K. M. Garadkar, S. A. Vanalakar, P. D. Lokhande, R. Sasikala, and P. P. Hankare, J. Mater. Sci.-Mater. Electron. 27, 825 (2016).
K. M. Farhan, A. H. Ansari, M. Hameedullah, E. Ahmad, F. M. Husain, Q. Zia, and U. Baig, Sci. Rep. 6, 27689 (2016).
R. M. Alwan, Q. A. Kadhim, K. M. Sahan, R. A. Ali, R. J. Mahdi, N. A. Kassim, and A. N. Jassim, J. Nanosci. Nanotechnol. 5, 1 (2015).
R. Lorenzi, A. Paleari, N. V. Golubev, E. S. Ignateva, V. N. Sigaev, M. Niederberger, and A. Lauria, J. Mater. Chem. C 3, 41 (2015).
N. A. Samat and R. M. Nor, Ceram. Int. 39, S545 (2013).
F. Mirjalili, M. Hasmaliza, and L. C. Abdullah, Ceram. Int. 36, 1253 (2010).
S. Naghibi, M. A. F. Sani, and H. R. M. Hosseini, Ceram. Int. 40, 4193 (2014).
S. L. Isley and R. L. Penn, J. Phys. Chem. B 110, 15134 (2006).
X. Haiyan, Z. Ai, and L. Zhang, J. Phys. Chem. C 113, 16625 (2009).
C. Costa, C. Pinheiro, I. Henriques, and C. A. T. Laia, ACS Appl. Mater. Interfaces 4, 1330 (2012).
L. Zhang and Y. Wu, J. Nanomater. 2013, 1 (2013).
D. Prentice, M. L. Pantoya, and A. E. Gash, Energy Fuels 20, 2370 (2006).
M. Grujic-Brojcin, S. Armakovic, N. Tomic, B. Abra-movic, A. Golubovic, B. Stojadinovic, A. Kremenovic, B. Babic, Z. Dohcevic-Mitrovic, and M. Scepanovic, Mater. Charact. 88, 30 (2014).
C. Karunakaran, P. Vinayagamoorthy, and J. Jayabharathi, Superlatt. Microstruct. 64, 569 (2013).
K. L. Foo, U. Hashim, K. Muhammad, and C. H. Voon, Nanoscale Res. Lett. 9, 429 (2014).
V. N. Nikolic, M. Vasic, and M. M. Milic, Ceram. Int. 44, 21145 (2018).
V. N. Nikolic, M. M. Vasic, and D. Kisic, J. Solid State Chem. 275, 187 (2019).
V. N. Nikolic, M. M. Milic, J. D. Zdravkovic, and V. Spasojevic, Russ. J. Phys. Chem. A 93, 377 (2019).
V. N. Nikolic, M. Tadic, L. Kopanja, N. Cvjeticanin, and V. Spasojevic, Ceram. Int. 43, 3147 (2017).
C. Karunakaran, V. Rajeswari, and P. Gomathisankar, Mater. Sci. Semicond. Proc. 14, 133 (2011).
M. M. N. Ansari and S. Khan, Phys. B (Amsterdam, Neth.) 520, 21 (2017).
I. A. Rahman and V. Padavettan, J. Nanomater. 2012, 1 (2012).
T. Wang, S. H. Song, X. L. Wang, J. J. Chen, and M. L. Tan, J. Sol-Gel Sci. Technol. 85, 356 (2018).
Z. Li, B. Hou, Y. Xu, D. Wu, Y. Sun, W. Hu, and F. Deng, J. Solid State Chem. 178, 1395 (2005).
M. I. Zaki, G. A. Mekhemer, N. E. Fouad, T. C. Jagadale, and S. B. Ogale, Mater. Res. Bull. 45, 1470 (2010).
C. Liu, B. Zou, A. J. Rondinone, and Z. J. Zhang, J. Am. Chem. Soc. 123, 4344 (2001).
J. J. Beltran, C. A. Barrero, and A. Punnoose, J. Solid State Chem. 240, 30 (2016).
A. Romeiro, D. Freitas, M. E. Azenha, M. Canle, and H. D. Burrows, Photochem. Photobiol. Sci. 16, 935 (2017).
B. G. Trewyn, I. I. Slowing, S. Giri, H. T. Chen, and V. S. Y. Lin, Acc. Chem. Res. 40, 846 (2007).
Y. Lu, Y. Yin, B. T. Mayers, and Y. Xia, Nano Lett. 2, 183 (2002).
B. Manikandan, T. Endo, S. Kaneko, K. R. Murali, and R. John, J. Mater. Sci.-Mater. Electron. 29, 9474 (2018).
P. K. Deheri, V. Swaminathan, S. D. Bhame, Z. Liu, and R. V. Ramanujan, Chem. Mater. 22, 6509 (2010).
M. J. Pawar and A. D. Khajone, J. Chem. Pharm. Res. 4, 1880 (2012).
A. E. Danks, S. R. Hall, and Z. J. M. H. Schnepp, Mater. Horiz. 3, 91 (2016).
M. Alsawafta, Y. M. Golestani, T. Phonemac, S. Badilescu, V. Stancovski, and V. V. Truong, J. Electrochem. Soc. 161, H276 (2014).
J. D. Mackenzie and E. P. Bescher, Acc. Chem. Res. 40, 810 (2007).
S. Ramesh, J. V. Ramaclus, E. Mosquera, and B. B. Das, RSC Adv. 6, 6336 (2016).
V. G. Kessler, J. Sol Gel Sci. Technol. 51, 264 (2009).
Z. H. Xiao, S. H. Jin, J. H. Wang, and C. H. Liang, Hyperfine Interact. 217, 151 (2013).
I. P. Prakash, N. Muralidharan, Nallamuthu, M. Venkteswarlu, and N. Satyanarayana, NSTI–Nanotech. 2, 115 (2005).
N. Rajic, M. Ceh, R. Gabrovsek, and V. Kaucic, J. Am. Ceram. Soc. 85, 1719 (2002).
ICSD Inorganic Crystals Structure Database (FIZ Karlsruhe, Eggenstein-Leopoldshafen, Germany, 2014), Vol. 2.
L. Lutterotti, Nucl. Instrum. Methods Phys. Res., Sect. B 268, 334 (2010).
A. C. Bent, Bulletin 130, 1 (1925).
D. Nicholls, Copper, Complexes and First-Row Transition Elements (Macmillan Education, UK, 1974), p. 281.
M. Meyn, K. Beneke, and C. Lagaly, Inorg. Chem. 32, 1209 (1993).
R. M. Cornell and R. Giovanoli, Polyhedron 7, 385 (1988).
U. Schwertmann and W. R. Fischer, Z. Anorg. Allgem. Chem. 346, 137 (1966).
Y. Cudennec and A. Lecerf, J. Solid State Chem. 179, 716 (2006).
R. Frison, G. Cernuto, A. Cervellino, O. Zaharko, G. M. Colonna, A. Guagliardi, and N. Masciocchi, Chem. Mater. 25, 4820 (2013).
S. P. Schwaminger, D. Bauer, P. Fraga-Garcia, F. E. Wagner, and S. Berensmeier, CrystEngComm. 19, 246 (2017).
L. E. Lagoeiro, J. Metamorph. Geol. 16, 415 (1998).
T. S. Gendler, V. P. Shcherbakov, M. J. Dekkers, A. K. Gapeev, S. K. Gribov, and E. McClelland, Geophys. J. Int. 160, 815 (2005).
K. F. McCarty, M. Monti, S. Nie, D. A. Siegel, E. Starodub, F. El Gabaly, A. H. McDaniel, A. Shavorskiy, T. Tyliszczak, H. Bluhm, N. C. Bartelt, and J. de la Figuera, J. Phys. Chem. C 118, 19786 (2014).
A. U. Gehring, H. Fischer, M. Louvel, K. Kunze, and P. G. Weidler, Geophys. J. Int. 179, 1361 (2009).
http://www1.lsbu.ac.uk/water/water_vibrational_spectrum.html. Accessed January 15, 2020.
K. Coenen, F. Gallucci, B. Mezari, E. Hensen, and M. van Sint Annaland, J. CO2 Util. 24, 228 (2018).
V. Yu. Dolmatova, I. I. Kulakova, V. Myllymakic, A. Vehanenc, A. A. Bochechkad, A. N. Panovad, and B. T. T. Nguyene, J. Superhard Mater. 38, 58 (2016).
L. M. Bronstein, X. Huang, J. Retrum, A. Schmucker, M. Pink, B. D. Stein, and B. Dragneam, Chem. Mater. 19, 3624 (2007).
http://lisa.chem.ut.ee/IR_spectra/conservation_materials/ethanol/. Accessed December 15, 2019.
E. R. Lippincott, A. van Valkenburg, C. E. Weir, and E. N. Bunting, J. Res. Natl. Bur. Stand. 61, 61 (1958).
H. A. Benessi and A. C. Jones, J. Phys. Chem. 63, 179 (1959).
Q. Hu, H. Suzuki, H. Gao, H. Araki, W. Yang, and T. Noda, Chem. Phys. Lett. 378, 299 (2003).
F. Iacona, G. Ceriola, and F. la Via, Mater. Sci. Semicond. Process. 4, 43 (2001).
X. Li, Z. Cao, Z. Zhang, and H. Dang, Appl. Surf. Sci. 252, 7856 (2006).
Q. Guo, D. Huang, X. Kou, W. Cao, L. Li, L. Ge, and J. Li, Ceram. Int. 43, 192 (2017).
T. Gholami, M. Salavati-Niasari, M. Bazarganipour, and E. Noori, Superlatt. Microstruct. 61, 33 (2013).
H. Yoshino, K. Kamiya, and H. Nasu, J. Non-Cryst. Solids 126, 68 (1990).
H. Kaya, D. Ngo, S. Gin, and S. H. Kim, J. Non-Cryst. Solids 52, 119722 (2020).
F. Rubio, J. Rubio, and J. L. Oteo, Spectrosc. Lett. 31, 199 (1998).
K. M. Davis and M. Tomozawa, J. Non-Cryst. Solids 201, 177 (1996).
R. H. Stolen and G. E. Walrafen, J. Chem. Phys. 64, 2623 (1976).
J. D. Mackenzie and S. A. Rice, Phys. Today 14, 62 (1961).
V. V. T. Padil and M. Cernik, Int. J. Nanomed. 8, 889 (2013).
M. A. Dar, S. H. Nam, Y. S. Kim, and W. B. Kim, J. Solid State Electrochem. 14, 1719 (2010).
J. Zhao, F. E. Huggins, Z. Feng, and G. P. Huffman, Clay Clay Miner. 42, 737 (1994).
M. D. P. Silva, F. C. Silva, F. S. M. Sinfronio, A. R. Paschoal, E. N. Silva, and C. W. A. Paschoal, J. Alloys Compd. 584, 573 (2014).
W. P. Gates, J. T. Kloprogge, J. Madejová, and F. Bergaya, Infrared and Raman Spectroscopies of Clay Minerals (Elsevier, Amsterdam, 2017), p. 1.
R. S. Pandurangi, M. S. Seehra, B. L. Razaboni, and P. Bolsaitist, Environ. Health Perspect. 86, 327 (1990).
A. P. Mirgorodskii, A. N. Lazarev, and I. P. Makarenko, Opt. Spectrosc. 29, 282 (1970).
B. P. Jelle, T.-N. Nilsen, P. J. Hovde, and A. Gustavsen, J. Build. Phys. 36, 99 (2012).
V. N. Nikolic, Magn. Nanomater. Electrocatal., Magnetochem.: Mater. Appl. 66, 34 (2020).
M. Catauro, F. Barrino, G. Dal Poggetto, G. Crescente, S. Piccolella, and S. Pacifico, Materials 13, 394 (2020).
A. Jitianu, M. Crisan, A. Meghea, I. Rau, and M. Zaharescu, J. Mater. Chem. 12, 1401 (2002).
G. Anbalagan, A. R. Prabakaran, and S. Gunasekaran, J. Appl. Spectrosc. 77, 95 (2010).
P. C. Schlecht and P. F. O’Connor, Third Supplement to NIOSH Manual of Analytical Methods (NMAM), 4th ed. (Natl. Inst. Occup. Safety and Health, 2003).
J. Hlavay, K. Jonas, S. Elek, and J. Inczedy, Clay. Clay Miner. 26, 139 (1978).
E. Nemecz and K. Rethy, Rep. Veszp. Univ. Chem. Eng. 5, 287 (1961).
L. B. Capeletti and J. H. Zimnoch, Fourier Transform Infrared and Raman Characterization of Silica-Based Materials, Applications of Molecular Spectroscopy to Current Research in the Chemical and Biological Sciences (IntechOpen, Rijeka, Croatia, 2016).
M. Cekerevac, M. Simicic, Lj. N. Bujanovic, and N. Popovic, Corros. Sci. 64, 204 (2012).
M. J. Song, S. W. Hwang, and D. Whang, Talanta 80, 1648 (2010).
J. Ping, S. Ru, K. Fan, J. Wu, and Y. Ying, Microchim. Acta 171, 117 (2010).
T. M. Nahir and E. F. Bowden, J. Electroanal. Chem. 410, 9 (1996).
H. H. Uhlig and J. R. Gilman, Corrosion 20, 289t (1964).
S. D. Giri and A. Sarkar, J. Electrochem. Soc. 163, H252 (2016).
J. Coates, Interpretation of Infrared Spectra, A Practical Approach, Encyclopedia of Analytical Chemistry: Applications, Theory and Instrumentation (Wiley, New York, 2007).
R. Bruckner, J. Non-Cryst. Solids 5, 123 (1970).
A. C. D. Chaklader and A. L. Roberts, J. Am. Ceram. Soc. 44, 35 (1961).
Funding
The research was funded by the Ministry of Education, Science and Technological Development of the Republic of Serbia.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Nikolić, V.N., Vasić, M.M., Milikić, J. et al. The Influence of Thermal Treatment on the Formation Mechanism of the Cu, Fe-Containing Nanocomposite Material Synthesized by the Sol–Gel Method. Phys. Solid State 63, 332–354 (2021). https://doi.org/10.1134/S1063783421020207
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1063783421020207