Abstract
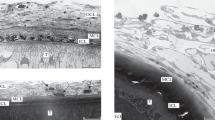
Published reports and the author’s own data concerning the morphology of glycocalyx on the acanthocephalan tegument surface have been analyzed. Successive formation of up to six glycocalyx modifications that differ with regard to dimensions and certain structural characteristics has been shown to occur during the life cycle of these worms. All modifications could apparently be subdivided into two groups that corresponded to “intestinal” and “tissue” acanthocephalan forms. Glycocalyx was the thickest in the tissue forms, namely, cystacanths from intermediate hosts and juvenile worms from paratenic hosts. This may be due to involvement of the glycocalyx in defense from the cellular reaction of the host organism.
Similar content being viewed by others
References
Amin, O.M., Heckmann, R.A., Mesa, R., and Mesa, E., Description and host relationships of cystacanths of Polymorphus spindlatus (Acanthocephala: Polymorphidae) from their paratenic fish hosts in Peru, J. Helminthol. Soc. Washington, 1995, vol. 62, no. 2, pp. 249–253.
Amin, O.M., Richard, A.H., Victor, I., and Vasquez, R., Immature Polyacanthorhynchus rhopalorhynchus (Acanthocephala: Polyacanthorhynchidae) in Venton, Hoplias malabaricus (Pisces) from Moca Vie River, Bolivia, with Notes in its Apical Organ and Histopathology, J. Helminthol. Soc. Washington, 1996, vol. 63, no. 1, pp. 115–119.
Barabashova, V.N., The structure and role of integuments of acanthocephalans (Acanthocephala) in their life activity, Parazitologiya, 1971, vol. 5, no. 5, pp. 446–454.
Baron, P.J., On the histology, histochemistry, and ultrastructure of the cysticercoid of Raillietina cesticillus (Molin, 1858) Fuhrmann, 1920 (Cestoda, Cyclophyllidea), Parasitology, 1971, vol. 62, no. 2, pp. 233–245.
Beermann, I., Arai, H.P., and Costerton, J.W., The ultrastructure of the lemnisci and body wall of Octospinifer macilentus (Acanthocephala), Can. J. Zool., 1974, vol. 52, no. 5, pp. 533–535.
Bennett, H.S., Morphological aspects of extracellular polysaccharides, J. Histochem. Cytochem., 1963, vol. 11, no. 1, pp. 14–23.
Bobrovskikh, E.Yu., The fine structure of the integument of mature acanthocephalans Echinorhynchus gadi (Acanthocephala), Parazitologiya, 1992, vol. 26, no. 5, pp. 396–401.
Bogoyavlenskii, Yu.K. and Ivanova, G.I., Mikrostruktura tkanei skrebnei (The Microstructure of Acanthocephalan Tissues), Moscow: Nauka, 1978.
Butterworth, P., The development of the body wall of Polymorphus minutus (Acanthocephala) in its intermediate host Gammarus pulex, Parasitology, 1969, vol. 59, no. 2, pp. 373–388.
Byram, J.E. and Fisher, F.M., The absorptive surface of Moniliformis dubius (Acanthocephala). 1. Fine structure, Tissue Cell, 1973, vol. 5, no. 4, pp. 553–579.
Calley, J., The functional significance of scolex retraction and subsequent cyst formation in the cysticercoid larva of hymenolepis microstoma, Parasitology, 1974, vol. 68, no. 2, pp. 207–227.
Chiang, C.-P. and Caulfield, J.P., Schistosoma mansoni: ultrastructural demonstration of a miracidial glycocalyx that cross-reacts with antibodies raised against the cercarial glycocalyx, Exp. Parasitol., 1988, vol. 67, no. 1, pp. 63–72.
Crompton, D.W.T., Morphological and histochemical observations on Polymorphus minutus (Goeze, 1782) with special reference to the body wall, Parasitology, 1963, vol. 53, nos. 3–4, pp. 663–7685.
Crompton, D.W.T. and Lee, D.L., The fine structure of the body wall of Polymorphus minutus (Goeze, 1782) (Acanthocephala), Parasitology, 1965, vol. 55, no. 2, pp. 357–364.
Dezfuli, B.S., Host–parasite interface between Asellus aquaticus (Isopoda) and larvae of Acanthocephalus anguillae (Acanthocephala), Fol. Parasitol., 2000, vol. 47, no. 2, pp. 154–156.
Dezfuli, B.S. and Giari, L., Amphipod intermediate host of Polymorphus minutus (Acanthocephala), parasite of water birds, with notes on ultrastructure of host-parasite interface, Fol. Parasitol., 1999, vol. 46, no. 2, pp. 117–122.
Dezfuli, B.S., Bosi, G., and Rossi, R., The ultrastructure of the capsule surrounding Pomphorhynchus laevis (Acanthocephala) in its intermediate host Echinogammarus stammeri (Amphipoda), Parassitilogia, 1992, vol. 34, pp. 61–69.
Dezfuli, B.S., Bosi, G., and Rossi, R., Fine structure of the envelope surrounding the cystacanth of Acanthocephalus clavula (Acanthocephala) in its intermediate host Echinogammarus stammeri (Amphipoda), Transact. Am. Microscop. Soc., 1994, vol. 113, no. 1, pp. 34–42.
Dezfuli, B.S., Simoni, E., Duclos, L., and Rossetti, E., Crustacean–acanthocephalan interaction and host cellmediated immunity: parasite encapsulation and melanization, Fol. Parasitol., 2008, vol. 55, no. 1, pp. 53–59.
Graeber, K. and Storch, V., Elektronmikroskopische und morphometrische Untersuchungen am Integument der Acanthocephala (Aschelminthes), Z. Parasitenk., 1978, vol. 57, no. 2, pp. 121–135.
Hammond, R.A., The fine structure of the trunk and praesoma wall of the Acanthocephalus ranae (Schrank, 1788), Luhe, 1911, Parasitology, 1967, vol. 57, no. 3, pp. 475–486.
Ito, S., Form and function of the glycocalyx on free cell surfaces, Trans. R. Soc. Lond. B, 1974, vol. 268, no. 891, pp. 55–66.
Klimova, T.V. and Nikishin, V.P., The first data on tissue organization of the parasite Acanthocephalus tenuirostris, in Sistematika i ekologiya parazitov. Tr. Tsentra parazitologii (Systematics and Ecology of Parasites. Transactions of the Parasitology Center), Movsesyan, S.O., Ed., Moscow: KMK, 2014, vol. 48, pp. 122–124.
Krasnoshchekov, G.P. and Nikishin, V.P., Ultrastructure of the cyst wall of metacestodes Aploparaksis polystictae Schiller, 1955 and A. furcigera (Cestoda, Cyclophyllidea), Parazitologiya, 1979a, vol. 1979, no. 13, pp. 3–250.
Krasnoshchekov, G.P. and Nikishin, V.P., The ultrastructure of protective envelopes of cestode larvae, in Ekologiya i morfologiya gel’mintov pozvonochnykh Chukotki (Ecology and Morphology of Helminths of Vertebrates of Chukotka), Sonin, M.D., Ed., Moscow: Nauka, 1979b, pp. 116–132.
Krasnoshchekov, G.P. and Nikishin, V.P., Adaptive role of helminth glycocalyx, in IV Vsesoyuz. simp. po parazitam i boleznyam vodnykh bespozvonochnykh. Tez. dokl. (Abstr. IV All-Union Symp. on Parasites and Diseases of Aquatic Invertebrates), Moscow: MGU, 1986, pp. 69–71.
Krasnoshchekov, G.P., Nikishin, V.P., and Pluzhnikov, L.T., Ultrastructure of the cyst wall of monocercus-type cestode larvae, Parazitologiya, 1983, vol. 17, no. 5, pp. 391–396.
Lackie, J.M. and Rotheram, S., Observations on the envelope surrounding Moniliformis dubius (Acanthocephala) in the intermediate host, Periplaneta americana, Parasitology, 1972, vol. 65, no. 2, pp. 303–308.
Lange, H., Über Struktur und Histochemie des Integuments von Echinorhynchus gadi Muller (Acanthocephala), Z. Zellforsch. Microscop. Anat., 1970, vol. 104, no. 1, pp. 149–164.
Lumsden, R.D., Cytological studies on the absorptive surface of cestodes. 1. The fine structure of the strobillar integument, Z. Parasitenk., 1966, vol. 27, no. 4, pp. 355–382.
Lumsden, R.D., Relationship of extrinsic polysaccharides to the tegument glycocalyx of cestodes, J. Parasitol., 1974, vol. 60, no. 2, pp. 374–375.
Marchand, B. and Grita-Timoulali, Z., Comparative ultrastructural study of the cuticle of larvae and adults of Centrorhynchus milvus Ward, 1956 (Acanthocephala, Centrorhynchidae), J. Parasitol., 1992, vol. 78, no. 2, pp. 355–359.
Nanduri, J., Dennis, J.E., Rosenberrys, T.L., Mahmoud, A.A.F., and Tartakoff, A.M., Glycocalyx of bodies versus tails of Schistosoma mansoni cercariae (lectin-binding, size, charge, and electron microscopic characterization), J. Biol. Chem., 1991, vol. 266, no. 2, pp. 1341–1347.
Nicholas, W.L. and Mercer, E.H., The ultrastructure of the tegument of Moniliformis dubius (Acanthocephala), Quart. J. Microscop. Sci., 1965, vol. 106, no. 2, pp. 137–146.
Nikishin, V.P., Covering tissue ultrastructure of late acanthella Arhythmorhynchus petrochenkoi (Schmidt, 1969) (Acanthocephala: Polymorphidae), Parazitologiya, 1985, vol. 19, no. 4, pp. 306–313.
Nikishin, V.P., The fine structure of the metasoma wall of the cystacanth Polymorphus strumosoides Lundström, 1942 (Acanthocephala: Polymorphidae), Parazitologiya, 1986, vol. 20, no. 5, pp. 403–408.
Nikishin, V.P., Formation of the capsule around Filicollis anatis in its intermediate host, J. Parasitol., 1992, vol. 78, no. 1, pp. 127–137.
Nikishin, V.P., Tsitomorfologiya skrebnei (Cytomorphology of Acanthocephalans), Moscow: GEOS, 2004.
Nikishin, V.P., Structure and differentiation of cysticercoid tissues. 3. Differentiation of tissues of the endocyst and typical diplocyst of Aploparaksis bulbocirrus (Cestoda, Hymenolepididae), Zool. Bespozv., 2010, vol. 7, no. 2, pp. 159–176.
Nikishin, V.P., Convergence of the cyst in cysticercoids (Cestoda, Hymenolepidata) and the tegument in cystacanths (Acanthocephala), Biol. Bull. (Moscow), 2011, vol. 38, no. 2, pp. 195–202.
Nikishin, V.P., Morphofunctional diversity of glycocalyx in tapeworms, Biol. Bull. Rev., 2017, vol. 7, no. 2, pp. 160–178.
Nikishin, V.P. and Skorobrechova, E.M., Encapsulation of acanthocephalans Corynosoma sp. in two reservoir host species, Dokl. Biol. Sci., 2007, vol. 417, no. 4, pp. 462–464.
Nikishin, V.P. and Skorobrechova, E.M., Relationships of acanthocephalans with hosts (morphological aspect), Usp. Sovrem. Biol., 2015, vol. 135, no. 2, pp. 203–221.
Nikishin, V.P., Pluzhnikov, L.T., and Leonov, S.A., The ultrastructure of integument of the cystacanth Polymorphus magnus (Acanthocephala, Polymorphidae), Parazitologiya, 1994, vol. 28, no. 1, pp. 52–59.
Rotheram, S. and Crompton, D.W.T., Observations on the early relationship between Moniliformis dubius (Acanthocephala) and the haemocytes of the intermediate host, Periplaneta americana, Parasitology, 1972, vol. 64, no. 1, pp. 15–21.
Sharpilo, V.P., On the ability of acanthella of the genus Centrorhynchus (Acanthocephala, Giganthorhynchidae) to passage through the reservoir hosts, in Materialy k nauch. konf. Vsesoyuz. ob-va gel’mintologov (Proc. Sci. Conf. All- Union Soc. Helminthologists), Moscow: Izd. AN SSSR, 1965, part 4, pp. 312–317.
Sharpilo, V.P. and Salamatin, R.V., Paratenicheskii parazitizm: stanovlenie i razvitie kontseptsii. Istoricheskii ocherk, bibliografiya (Paratenic Parasitism: the Formation and Development of the Concept. Historical Essay and Bibliography), Kiev: Institut zoologii im. I.I. Shmal’gauzena NAN Ukrainy, 2005.
Skorobrechova, E.M., Morphology of the relationships of the acanthocephalan Corynosoma strumosum (Acanthocephales: Polymorphidae) and paratenic hosts in nature and experiment, Cand. Sci. (Biol.) Dissertation, St. Petersburg: ZIN RAN, 2014.
Skorobrechova, E.M. and Nikishin, V.P., Structure of capsule surrounding acanthocephalans Corynosoma strumpsum in paratenic hosts of three species, Parasitol. Res, 2011, vol. 108, no. 2, pp. 467–475.
Skorobrechova, E.M. and Nikishin, V.P., Encapsulation of the acanthocephalan Corynosoma strumosum in the fish Hemichromis bimaculatus: a preliminary experimental study, Vestn. SVNTs DVO RAN, 2012, no. 3, pp. 52–58.
Skorobrechova, E.M. and Nikishin, V.P., Dependence of the structure of the capsule surrounding the acanthocephalan Corynosoma strumosum on the species of its natural paratenic host, Biol. Bull. (Moscow), 2013, vol. 41, no. 6, pp. 333–348.
Skorobrechova, E.M. and Nikishin, V.P., The morphological peculiarities of the acanthocephalan Corynosoma strumosum (Rudolphi, 1802) (Polymorphidae) in paratenic hosts, the eelpout Zoarces elongatus (Kner, 1868) (Zoarcidae) and the halibut Hippoglossus stenolepis (Schmidt, 1904) (Pleuronectidae), Russ. J. Mar. Biol., 2017, vol. 43, no. 1, pp. 49–56.
Skorobrechova, E., Nikishin, V., and Lisitsyna, O., Structure of capsule around acanthocephalan Corynosoma strumosum from uncommon paratenic hosts—lizards of two species, Parasitol. Res., 2012, vol. 110, no. 1, pp. 459–467.
Taraschewski, H., Host-parasite interactions in Acanthocephala: a morphological approach, Adv. Parasitol., 2000, vol. 46, pp. 1–179.
Valkounova, J., Comparative studies on the morphology, histology and histochemistry of metacestodes (Hymenolepididae, Dilepididae and Dipylididae), Fol. Parasitol., 1987, vol. 34, no. 2, pp. 117–128.
Weinbaum, S., Tarbell, J.M., and Damiano, E.R., The structure and function of the endothelial glycocalyx layer, Annu. Rev. Biomed. Eng., 2007, vol. 9, pp. 121–167.
Whitfield, P.J., Acanthocephala, in Biology of the Integument, vol. 1: Invertebrates, Richards, K.S., Ed., Berlin: Springer Verlag, 1984, pp. 234–241.
Wright, R.D. and Lumsden, R.D., Ultrastructural and histochemical properties of the acanthocephalan epicuticle, J. Parasitol., 1968, vol. 54, no. 6, pp. 1111–1123.
Wright, R.D. and Lumsden, R.D., The acanthor tegument of Moniliformis dubius, J. Parasitol., 1970, vol. 56, no. 4, pp. 727–735.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © V.P. Nikishin, 2018, published in Izvestiya Akademii Nauk, Seriya Biologicheskaya, 2018, No. 1, pp. 42–54.
Rights and permissions
About this article
Cite this article
Nikishin, V.P. Glycocalyx Modifications in Acanthocephalans. Biol Bull Russ Acad Sci 45, 35–46 (2018). https://doi.org/10.1134/S1062359018010090
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1062359018010090