Abstract
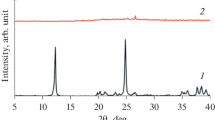
Two procedures were suggested for preparing a micro-mesoporous zeolite of MTT topology (ZSM-23 type) with the molar ratio SiO2/Al2O3 = 100 in the presence of dimethylformamide as a template. The first procedure involves hydrothermal crystallization under the conditions ensuring the formation of nanosized crystals. The second procedure is postsynthetic treatment of the zeolite with a NaOH solution to increase the mesopore volume. Samples of ZSM-23 zeolites with the crystal size of 100–200 nm with improved textural characteristics (micropore volume up to 0.1 cm3 g–1, mesopore volume up to 0.70 cm3 g–1) were obtained in >85% yield based on the silica used. The acid characteristics of the synthesized samples are similar to those of the zeolites prepared using pyrrolidine, which is expensive and is not produced in Russia.





Similar content being viewed by others
REFERENCES
Vogt, E.T.C., Whiting, G.T., Chowdhury, A.D., and Weckhuysen, B.M., Adv. Catal., 2015, vol. 58, p. 143. https://doi.org/10.1016/bs.acat.2015.10.001
Gerasimov, D.N., Fadeev, V.V., Loginova, A.N., and Lysenko, S.V., Catal. Ind., 2015, vol. 7, no. 2, p. 128. https://doi.org/10.1134/S2070050415020051
Deldari, H., Appl. Catal. A: General, 2005, vol. 293, p. 1. https://doi.org/10.1016/j.apcata.2005.07.008
Chen, Х., Xi, H., Lin, M., Jia, L., Hou, B., Li, D., and Niu, P., Int. J. Hydrogen Energy, 2019, vol. 44, p. 19762.
Kumar, R. and Ratnasamy, P., J. Catal., 1989, vol. 116, p. 440. https://doi.org/10.1016/0021-9517(89)90110-3
Nicholas, C.P., Appl. Catal. A: General, 2017, vol. 543, p. 82. https://doi.org/10.1016/j.apcata.2017.06.011
Piryutko, L.V., Chernyavskii, V.S., Lysikov, A.I., Kharitonov, A.S., and Noskov, A.S., Russ. J. Appl. Chem., 2018, vol. 91, no. 12, p. 2030. https://doi.org/10.1134/S1070427218120157
Thomas, J.M., Millward, G.R., White, D., and Ramdas, S., J. Chem Soc., Chem. Commun., 1988, p. 434.
Parker, L. and Bibby, D., Zeolites, 1983, vol. 3, p. 8. https://doi.org/10.1016/0144-2449(83)90078-7
Araya, A. and Lowe, B.M., US Patent 4705674, 1985.
Plank, C.J., Rosinski, E.J., and Rubin, M.K., US Patent 4076842, 1978.
Ahmad, M., US Patent 5332566, 1994.
Ojo, A.F., Zhang, Y., Lei, G.-D., and Zones, S., US Patent 9573124, 2017.
Kameshkov, A.V. and Gaile, A.A., Khim. Khim. Tekhnol. Izv. Sankt-Peterb. Gos. Tekhnol. Inst. (Tech. Univ.), 2015, no. 29, p. 49. https://doi.org/10.15217/issn998984-9.2015.29.49
Deldari, H., Appl. Catal. A: General, 2005, vol. 293, p. 1. https://doi.org/10.1016/j.apcata.2005.07.008
Archer, R.H., Zones, S., and Davis, M.E., Micropor. Mesopor. Mater., 2010, vol. 130, p. 255. https://doi.org/10.1016/j.micromeso.2009.11.018
Liu, Y., Wang, Z., Ling, Y., Li, X., Liu, Y., and Wu, P., Chin. J. Catal., 2009, vol. 30, p. 525. https://doi.org/10.1016/S1872-2067(08)60115-1
Piryutko, L.V., Parfenov, M.V., Lysikov, A.I., and Gerasimov, E.Yu., Russ. J. Appl. Chem., 2019, vol. 92, no. 12, p. 1664.
Wang, B., Tian, Z., Li, P., and Lin, L., Micropor. Mesopor. Mater., 2010, vol. 134, p. 203. https://doi.org/10.1016/j.micromeso.2010.06.001
Holm, M.S., Taarning, E., Egeblad, K., and Christensen, C.H., Catal. Today, 2011, vol. 168, p. 3. https://doi.org/10.1016/j.cattod.2011.01.007
Ivanova, I.I., Kuznetsov, A.S., Knyazeva, E.E., Fajula, F., Thibault-Starzyk, F., Fernandez, C., and Gilson, J.P., Catal. Today, 2011, vol. 168, p. 133. https://doi.org/10.1016/j.cattod.2010.11.091
Van Donk, S., Janssen, A.H., Bitter, J.H., and de Jong, K.P., Catal. Rev., 2003, vol. 45, p. 297. https://doi.org/10.1081/CR-120023908
Perez-Ramírez, J., Christensen, C.H., Egeblad, K., Christensen, C.H., and Groen, J.C., Chem. Soc. Rev., 2008, vol. 37, p. 2530. https://doi.org/10.1039/B809030K
Silva, B.J.B., Sousa, L.V., Sarmento, L.R.A., Carvalho, R.P., Quintela, P.H.L., Pacheco, J.G.A., Fréty, R., and Silvaa, A.O.S., Micropor. Mesopor. Mater., 2019, vol. 290, p. 1. https://doi.org/10.1016/j.micromeso.2019.109647
Moller, K. and Bein, T., Chem. Soc. Rev., 2013, vol. 42, p. 3689. https://doi.org/10.1039/c3cs35488a
Valtchev, V. and Tosheva, L., Chem. Rev., 2013, vol. 113, no. 8, p. 6734. https://doi.org/10.1021/cr300439k
Knyazeva, E.E., Dobryakova, I.V., Shkuropatov, A.V., Ponomareva, O.A., Kolyagin, Yu.G., and Ivanova, I.I., Russ. J. Appl. Chem., 2018, vol. 91, no. 11, p. 1821. https://doi.org/10.1134/S1070427218110125
Ahmed, M.H.M., Muraza, O., Al-Amer, A.M., and Yamani, Z.H., Micropor. Mesopor. Mater., 2016, vol. 227, p. 48.
Zhai, M., Li, L., Ba, Y., Zhu, K., and Zhou, X., Catal. Today, 2019, vol. 329, p. 82.
Zones, S.I., US Patent 5053373, 1991.
Wu, Q., Wang, X., Meng, X., Yang, C., Liu, Y., Jin, Y., Yang, Q., and Xiao, F.S., Micropor. Mesopor. Mater., 2014, vol. 186, p. 106. https://doi.org/10.1016/j.micromeso.2013.11.043
Treacy, M.M.J. and Higgins, J.B., Collection of Simulated XRD Powder Patterns for Zeolites, Elsevier, 2007, 5th ed., p. 300.
Smirnova, M.Yu., Piryutko, L.V., Brester, Yu.S., Parfenov, M.V., Kaichev, V.V., Klimov, O.V., and Noskov, A.S., Petrol. Chem., 2020, vol. 60, no. 2, p. 212. https://doi.org/10.1134/S0965544120020085
Piryutko, L.V., Lazareva, S.V., Chernyavskii, V.S., Kharitonov, A.S., and Noskov, A.S., Petrol. Chem., 2019, vol. 59, no. 7, p. 726. https://doi.org/10.1134/S0965544119070144
Funding
The study was performed within the framework of the government assignment for the Boreskov Institute of Catalysis, SB RAS (project AAAA-A21-121011890074-4).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest requiring disclosure in this article.
Additional information
Translated from Sovremennye Molekulyarnye Sita. Advanced Molecular Sieves, 2021, Vol. 3, No. 1, pp. 27–35.
Rights and permissions
About this article
Cite this article
Pirutko, L.V., Parfenov, M.V., Lysikov, A.I. et al. Synthesis of Micro-Mesoporous ZSM-23 Zeolite. Pet. Chem. 61, 276–283 (2021). https://doi.org/10.1134/S0965544121020080
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0965544121020080