Abstract
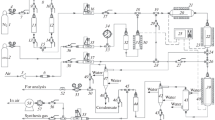
The process of partial catalytic oxidation of a propane–butane mixture with atmospheric oxygen in a pilot unit has been accomplished. Comparative tests with α-Al2O3-supported nickel catalysts and a pressed metal wire catalyst (PMC) made from 12Kh18N10Т steel wire of a 0.2 mm diameter have been conducted. In both cases, byproducts (СН4, СО2, and coke) are formed along with the target products (Н2, СО). It has been shown that the compositions of the resulting synthesis gas are close; however, the Н2/CO molar ratio is closer to 2 in the case of PMC catalyst, which meets the requirements of the subsequent Fischer–Tropsch synthesis. Problems associated with coking and complete degradation of the structure of the α-Al2O3-based catalysts have been revealed and explained in terms of the low efficiency of heat removal from the surface and the formation of carbon in micropores followed by its expansion. These phenomena are characteristic of 12Kh18N10T PMC to a substantially lesser extent and have a local character, suggesting that this catalyst holds promise for its further investigation.
Similar content being viewed by others
References
Kh. M. Minachev, N. Ya. Usachev, V. N. Udut, and Yu. S. Khodakov, Usp. Khim. 57, 385 (1988).
V. S. Arutyunov and O. V. Krylov, Organic Chemistry: Oxidative Transformations of Methane (Yurait, Moscow, 2017) [in Russian].
V. S. Arutyunov, V. I. Savchenko, and I. V. Sedov, Neftegazokhimiya, No. 4, 14 2016.
A. I. Belousov, E. A. Izzheurov, and A. D. Setin, Vibrational Strength and Reliability of Flying-Vehicle Engines and Systems, Ed. by A. I. Belousov (KAI, Kuibyshev, 1975), Iss. 2, p. 70 [in Russian].
M. M. Levitan and O. G. Rasin, in Heat and Mass Transfer in Systems with Porous Elements: Collection of Articles (Minsk, 1981), p. 91 [in Russian].
A. M. Zhizhkin and A. I. Belousov, in Mathematical Modeling of Information Processes and Systems in Science, Technology, and Society: Collection of Scientific Papers (Samara, 2004), p. 43 [in Russian].
Y. F. Chang and H. Heinemann, J. Catal. Lett. 215, 58 (1993).
D. Dissanayake, M. P. Rosynek, and J. H. Lunsford, J. Phys. Chem. 97, 36 (1993).
W. J. M. Vermeiren, E. Blomsma, and P. A. Jacobs, Catal. Today, 13, 427 (1992).
Y. Zhang, G. Xiong, S. Sheng, and W. Yang, Catal. Today 63, 517 (2000).
J. B. Claridge, M. L. H. Green, S. C. Tsang, et al., Catal. Lett. 22, 299 (1993).
A. A. Mel’nikov, S. A. Filipchenko, E. A. Maznitsyna, and A. Yu. Emeneva, Izv. Samarsk. Nauchn. Tsentra Ross. Akad. Nauk 17, 472 (2015).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © A.S. Mityugin, S.A. Filipchenko, 2017, published in Neftekhimiya, 2017, Vol. 57, No. 6, pp. 651–655.
Rights and permissions
About this article
Cite this article
Mityugin, A.S., Filipchenko, S.A. Partial Catalytic Oxidation of C3–C4 Hydrocarbons on Pilot Scale. Pet. Chem. 57, 1031–1035 (2017). https://doi.org/10.1134/S0965544117120088
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0965544117120088