Abstract
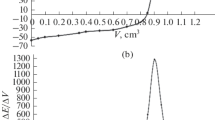
A novel method of catalytic thermometric titration has been developed for the determination of the acidity of oils using titration with KOH in isopropanol as titrant. The amounts of oil, concentration and delivery rate of titrant, volumes and types of thermometric end-point indicator were investigated. The results show that mixture of acetone and chloroform used as indicator exhibits strongly exothermic effects to reflect end point obviously. The results obtained from the thermometric titration have good agreement with standard potentiometric and visual titration methods. The procedure is fast, easy to use, accurate, and highly reproducible to measure lower acidity in coloured oils. It is very suitable for the routine process and quality control of many types of oils.
Similar content being viewed by others
References
Q. J. He, Gz. Chem. Ind. 5, 17 (2013).
P. Borrull, V. Cerda, J. Guasch, and J. Torres, Thermochim. Acta. 98, 1 (1986).
E. J. Greenhow, Chem. Rev. 77, 835 (1977).
G. A. Vaughan and J. J. Swithenbank, Analyst 90, 594 (1965).
H. F. Ferguson, D. J. Frurip, and A. J. Pastor, Thermochim. Acta 363, 1 (2000).
P. E. G. Jean and M. R. José, J. Chem. Thermodyn. 55, 193 (2012).
L. Tao, H. Sun, and Y. Gong, Metall. Anal. 32, 46 (2012).
T. Jiang, H. Y. Zhan, and L. J. Wang, Light Metals 46, 22 (2011).
K. S. Thomas, J. Am. Oil. Chem. Soc. 80, 21 (2003).
J. D. Carneiro, M. A. Feres, and E. S. Godinho, J. Braz. Chem. Soc. 13, 692 (2002).
E. S. Oswaldo, C. B. Julio, and G. O. Neto, Analyst 118, 1453 (1993).
M. Kashanipour, S. Evans, L. A. Torrijos, E. J. Greenhow, and D. J. Roberts, Anal. Proc. 12, 436 (1986).
I. A. Micek, Catal. Lett. 81, 3 (2002).
M. R. D. Rivera, J. S. Matos, and F. Socorro, J. Therm. Anal. Calorim. 92, 79 (2008).
A. Demoz and R. J. Mikula, Eng. Geol. 117, 12 (2011).
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Neftekhimiya, 2017, Vol. 57, No. 6, pp. 720–724.
The article is published in the original.
Rights and permissions
About this article
Cite this article
Hu, JQ., Yang, SZ., Zhang, JJ. et al. The Determination of Lower Acidity in Several Coloured Oils by Catalyzed Thermometric Titration. Pet. Chem. 57, 1099–1104 (2017). https://doi.org/10.1134/S0965544117120052
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0965544117120052