Abstract
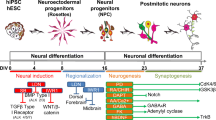
Introduction. The study of neuronal differentiation of human induced pluripotent stem cells (iPSCs) offers wide prospects for modeling and analyzing the pathogenesis of human neurodegenerative brain diseases, screening the drugs for efficacious treatment, and obtaining specific cell material for personalized neurotransplantation.
The aim of this study
was to examine the ultrastructural properties of iPSCs reprogrammed from healthy donor fibroblasts and differentiated into ventral mesencephalic neurons on day 7, 14 and 19 in vitro.
Materials and methods
. We used a previously obtained iPSC cell line from a healthy donor. Cell differentiation was performed according to a previously designed protocol with modifications. Ultrathin sections (50–70 nm) of cultures embedded in Epon were contrasted with uranyl acetate and lead citrate, and then examined with the JEOL JEM-1011 transmission electron microscope (Japan).
Results
. By day 19 in vitro, the study material contained cells, most of which were very similar in their fine structure to mature neurons: they contained the Golgi apparatus and emerging Nissl bodies, and had formed various junctions with each other, including symmetric, asymmetric and mixed avesicular contacts, which preceded the formation of mature chemical synapses. An important ultrastructural criterion for synaptic development and maturation was the appearance of large granular vesicles, corresponding to “transport packages” necessary for the construction of the synaptic active zone and involving in the formation and differentiation of both postsynaptic and presynaptic structures.
Conclusion
s. Our results suggest that ultrastructural changes in iPSCs differentiable into neurons, in the early stages of cultivation, reproduce the changes observed in early embryogenesis of the human brain, with their cellular composition resembling a neural tube containing mitotic neuroepithelial cells, radial glia, and maturing neurons. In the future, ultrastructural study of changes in iPSCs development, obtained from patients and undergoing neuronal differentiation after genome editing, will allow us to morphologically assess the degree of genetic defect elimination in transplantable cells.





Similar content being viewed by others
REFERENCES
Novosadova, E.V. and Grivennikov, I.A., Induced pluripotent stem cells: from production to application in biochemical and biomedical researches, Usp. Biol. Khim., 2014, vol. 54, pp. 3–38.
Vetchinova, A.S., Konovalova, E.V., Lunev, E.A., and Illarioshkin, S.N., A genome editing technology and capabilities of its application in cellular neurobiology, Ann. Clin. Exp. Neurol., 2014, vol. 9, no. 4, pp. 59–64.
Capetian, P., Müller, L., Volkmann, J., et al., Visualizing the synaptic and cellular ultrastructure in neurons differentiated from human induced neural stem cells—an optimized protocol, Int. J. Mol. Sci., 2020, vol. 21, no. 5, p. 1708. https://doi.org/10.3390/ijms21051708
Novosadova, E.V., Manuilova, E.S., Arsenyeva, E.L., et al., Fibroblast-like cells as an effective feeder for the cultivation and derivation of new lines of human induced pluripotent stem cells, Dokl. Biochem. Biophys., 2016, vol. 470, no. 1, pp. 353–356. https://doi.org/10.1134/S1607672916050136
Lebedeva, O.S., Novosadova, E.V., Manuilova, E.S., et al., Obtaining and characterization of a cell model of Parkinson’s disease based on induced pluripotent stem cells, in Stvolovye kletki i regenerativnaya meditsina (Stem Cells and Regenerative Medicine), Moscow: Mosk. Gos. Univ., 2014, pp. 158–168.
Hu, B.Y., Weick, J.P., Yu, J., et al., Neural differentiation of human induced pluripotent stem cells follows developmental principles but with variable potency, Proc. Natl. Acad. Sci. U.S.A., 2010, vol. 107, no. 9, pp. 4335–4340. https://doi.org/10.1073/pnas.0910012107
Mariani, J., Simonini, M.V., Palejev, D., Tomasini, L., et al., Modeling human cortical development in vitro using induced pluripotent stem cells, Proc. Natl. Acad. Sci. U.S.A., 2012, vol. 109, no. 31, pp. 12770–12775. https://doi.org/10.1073/pnas.1202944109
Paşca, A.M., Sloan, S.A., Clarke, L.E., et al., Functional cortical neurons and astrocytes from human pluripotent stem cells in 3D culture, Nat. Methods, 2015, vol. 12, no. 7, pp. 671–678. https://doi.org/10.1038/nmeth.3415
Tao, Y. and Zhang, S.C., Neural subtype specification from human pluripotent stem cells, Cell Stem Cell, 2016, vol. 19, no. 5, pp. 573–586. https://doi.org/10.1016/j.stem.2016.10.015
Salikhova, D.I., Fedyunina, I.A., Bukharova, T.B., et al., Key stages of IPSC differentiation into neuronal and glial cells, Genes Cells, 2018, vol. 13, no. 3, pp. 52–55. https://doi.org/10.23868/201811033
Perrier, A.L., Tabar, V., Barberi, T., et al., Derivation of midbrain dopamine neurons from human embryonic stem cells, Proc. Natl. Acad. Sci. U.S.A., 2004, vol. 101, pp. 12543–12548. https://doi.org/10.1073/pnas.0404700101
Espuny-Camacho, I., Michelsen, K.A., Gall, D., et al., Pyramidal neurons derived from human pluripotent stem cells integrate efficiently into mouse brain circuits in vivo, Neuron, 2013, vol. 77, no. 3, pp. 440–456. https://doi.org/10.1016/j.neuron.2012.12.011
Eiraku, M., Watanabe, K., Matsuo-Takasaki, M., et al., Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals, Cell Stem Cell, 2008, vol. 3, no. 5, pp. 519–532. https://doi.org/10.1016/j.stem.2008.09.002
Brooks, P.T., Rasmussen, M.A., and Hyttel, P., Structural analysis of three-dimensional human neural tissue derived from induced pluripotent stem cells, J. Stem Cell Res. Ther., 2016, vol. 6, p. 337. https://doi.org/10.4172/2157-7633.1000337
Pereda, A.E., Electrical synapses and their functional interactions with chemical synapses, Nat. Rev. Neuros-ci., 2014, vol. 15, no. 4, pp. 250–263. https://doi.org/10.1038/nrn3708
Maksimova, E.V., The main stages of nerve cell differentiation, in Neiroontogenez (Neuroontogenesis), Moscow: Nauka, 1985, pp. 6–77.
Bogolepov, N.N., Yakovleva, N.I., Frumkina, L.E., and Koroleva, S.K., Various types of non-synaptic intercellular contacts in the developing rat brain, Arkh. Anat., Gistol. Embriol., 1986, vol. 90, no. 2, pp. 45–53.
Wenisch, S., Trinkaus, K., Hild, A., et al., Immunochemical, ultrastructural and electrophysiological investigations of bone-derived stem cells in the course of neuronal differentiation, Bone, 2006, vol. 38, no. 6, pp. 911–921. https://doi.org/10.1016/j.bone.2005.10.021
Frumkina, L.E. and Khaspekov, L.G., Contemporary conceptions about the development of chemical synapses and molecular mechanisms of synaptogenesis in the central nervous system, Neurochem. J., 2003, vol. 20, no. 3, pp. 165–178.
Ahmari, S.E., Buchanan, J., and Smith, S.J., Assembly of presynaptic active zones from cytoplasmic transport packets, Nat. Neurosci., 2000, vol. 3, no. 5, pp. 445–451. https://doi.org/10.1038/74814
Garner, C.C., Zhai, R.G., Gundelfinger, E.D., and Ziv, N.E., Molecular mechanisms of CNS synaptogenesis, Trends Neurosci., 2002, vol. 25, no. 5, pp. 243–251. https://doi.org/10.1016/s0166-2236(02)02152-5
Ziv, N.E. and Garner, C.C., Principles of glutamatergic synapse formation: seeing the forest for the trees, Curr. Opin. Neurobiol., 2001, vol. 11, no. 5, pp. 536–543. https://doi.org/10.1016/s0959-4388(00)00246-4
Ray, B., Chopra, N., Long, J.M., and Lahiri, D.K., Human primary mixed brain cultures: preparation, differentiation, characterization and application to neuroscience research, Mol. Brain, 2014, vol. 7, p. 63. https://doi.org/10.1186/s13041-014-0063-0
Fletcher, T.L., De Camilli, P., and Banker, G., Synaptogenesis in hippocampal cultures: evidence indicating that axons and dendrites become competent to form synapses at different stages of neuronal development, J. Neurosci., 1994, vol. 14, no. 11, pp. 6695–6706. https://doi.org/10.1523/JNEUROSCI.14-11-06695.1994
Matteoli, M., Verderio, C., Krawzeski, K., et al., Mechanisms of synaptogenesis in hippocampal neurons in primary culture, J. Physiol. (Paris), 1995, vol. 89, no. 1, pp. 51–55. https://doi.org/10.1016/0928-4257(96)80551-1
Vicario-Abejón, C., Collin, C., McKay, R.D., and Segal, M., Neurotrophins induce formation of functional excitatory and inhibitory synapses between cultured hippocampal neurons, J. Neurosci., 1998, vol. 18, no. 18, pp. 7256–7271. https://doi.org/10.1523/JNEUROSCI.18-18-07256.1998
Zhang, Z.N., Freitas, B.C., Qian, H., et al., Layered hydrogels accelerate iPSC-derived neuronal maturation and reveal migration defects caused by MeCP2 dysfunction, Proc. Natl. Acad. Sci. U.S.A., 2016, vol. 113, no. 12, pp. 3185–3190. https://doi.org/10.1073/pnas.1521255113
Waitt, A.E., Reed, L., Ransom, B.R., and Brown, A.M., Emerging roles for glycogen in the CNS, Front. Mol. Neurosci., 2017, vol. 10, p. 73. https://doi.org/10.3389/fnmol.2017.00073
Pannese, E., Neurocytology: Fine Structure of Neurons, Nerve Processes, and Neuroglial Cells, New York: Springer-Verlag, 1994.
Massa, P.T. and Mugnaini, E., Cell junctions and intramembrane particles of astrocytes and oligodendrocytes: a freeze-fracture study, Neuroscience, 1982, vol. 7, no. 2, pp. 523–538. https://doi.org/10.1016/0306-4522(82)90285-8
Ge, W.P., Zhou, W., Luo, Q., et al., Dividing glial cells maintain differentiated properties including complex morphology and functional synapses, Proc. Natl. Acad. Sci. U.S.A., 2009, vol. 106, no. 1, pp. 328–333. https://doi.org/10.1073/pnas.0811353106
Pamies, D., Barreras, P., Block, K., et al., A human brain microphysiological system derived from induced pluripotent stem cells to study neurological diseases and toxicity, ALTEX, 2017, vol. 34, no. 3, pp. 362–376. https://doi.org/10.14573/altex.1609122
Ke, Q., Li, L., Yao, X., et al., Enhanced generation of human induced pluripotent stem cells by ectopic expression of Connexin 45, Sci. Rep., 2017, vol. 7, no. 1, p. 458. https://doi.org/10.1038/s41598-017-00523-y
Todd, K.L., Kristan, W.B., and French, K.A., Gap junction expression is required for normal chemical synapse formation, J. Neurosci., 2010, vol. 30, no. 45, pp. 15277–15285. https://doi.org/10.1523/JNEUROSCI.2331-10.2010
Kelava, I. and Lancaster, M.A., Dishing out mini-brains: current progress and future prospects in brain organoid research, Dev. Biol., 2016, vol. 420, no. 2, pp. 199–209. https://doi.org/10.1016/j.ydbio.2016.06.037
Funding
Russian Science Foundation Grant no. 19-15-00320.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that there are no clear or potential conflicts of interest associated with the publication of this article.
Rights and permissions
About this article
Cite this article
Kutukova, K.A., Frumkina, L.E., Ivanov, M.V. et al. Ultrastructural Organization of Ventral Mesencephalic Neurons Derived from Human Induced Pluripotent Stem Cells. Hum Physiol 46, 886–894 (2020). https://doi.org/10.1134/S0362119720080071
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0362119720080071