Abstract
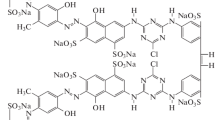
In this paper, the sol–gel method has been use to synthesized TiO2/Ag2O nanoparticles for photocatalytic degradation of azo dye Acid Red 18 (AR18) in aqueous solution. The TiO2/Ag2O nanoparticles were characterized by scanning electron microscopy (SEM), X-ray diffraction (XRD), Fourier transform infrared (FT-IR), and Brunauer–Emmett–Teller (BET) surface area analysis. A photoreactor with capacity 0.5 L, equipped with a low mercury pressure lamp UV-C (15 W) was used. The effective factors for the degradation of dye were determined and optimized using Taguchi fractional design method with four factors having four levels for each factor. Analysis the response of each experiment was based average standard deviation values was calculated. The Taguchi results showed that pH 3, catalyst amount 45 mg/L, H2O2 concentration 25 ppm and temperature 40°C was optimum conditions for this reaction. The most influenced of each factor on the process determined using Analysis of Variance (ANOVA) method. The most significant factor in this process was pH. The interaction between catalyst amount and temperature was the most influencing interaction. So first order reaction with k = 0.0289 min–1 was observed for the photocatalytic degradation reaction.













Similar content being viewed by others
REFERENCES
H. Zollinger, Color Chemistry Synthesis, Properties, and Applications of Organic Dyes and Pigments, 3nd ed. (Wiley-VCH, Weinheim, 2003).
D. R. Waring and G. Hallas, The Chemistry and Application of Dyes (Plenum, New York, 1990).
L. Ai, C. Zhang, F. Liao, Y. Wang, M. Li, L. Meng, and J. Jiang, J. Hazard. Mater. 198, 282 (2011).
N. Bao, Y. Li, Z. T. Wei, G. B. Yin, and J. J. Niu, J. Phys. Chem. C 115, 5708 (2011).
M. Torkaman, R. Moradi, and B. Keyvani, Rev. Roum. Chim. 61, 763 (2016).
S. Gul and O. Ozcan, Chem. Eng. J. 155, 684 (2009).
V. Augugliaro, V. Loddo, M. Pagliaro, G. Palmisano, and L. Palmisano, Clean by Light Irradiation Practical Applications of Supported TiO 2 (RSC, Cambridge, UK, 2010).
M. Nikazar, K. Gholivand, and K. Mahanpoor, Kinet. Catal. 48, 230 (2007).
C. G. Silva and J. L. Faria, J. Photochem. Photobiol., A 155, 133 (2003).
S. Taghavi Fardood, Z. Golfar, and A. Ramazani, J. Mater. Sci. 28, 17002 (2017).
N. M. Mahmoodi, Water Air Soil Pollut. 224, 1612 (2013).
B. Bayarri, J. Gimenez, D. Curo, and S. Esplugas, Catal. Today 101, 227 (2005).
C. Liu, C. Cao, X. Luo, and S. Luo, J. Hazard. Mater. 285, 319 (2015).
N. Sobana, K. Selvam, and M. Swaminathan, Sep. Purif. Technol. 62, 648 (2008).
M. Nikazar, K. Gholivand, and K. Mahanpoor, Desalination 219, 293 (2008).
J. Saien and A. R. Soleymani, J. Hazard. Mater. 144, 506 (2007).
C. Galindo, P. Jacques, and A. Kalt, J. Photochem. Photobiol., A 130, 35 (2003).
A. Fujishima, T. N. Rao, and D. A. Tryk, J. Photochem. Photobiol., C 1, 1 (2000).
H. Balavi, S. Samadanian-Isfahani, M. Mehrabani-Zeinabad, and M. Edrissi, Powder Technol. 249, 549 (2013).
M. A. H. Devadi, M. Krishna, H. N. Narasimha Murthy, and B. S. Sathyanarayana, Proc. Mater. Sci. 5, 612 (2014).
S. M. Mousavi, S. Yaghmaei, A. Jafari, M. Vossoughi, and Z. Ghobadi, Chem. Eng. Process 46, 935 (2007).
M. E. Olya, M. Vafaee, and M. Jahangiri, J. Saudi Chem. Soc. 21, 633 (2017).
R. Moradi, J. Hossieni, A. Bodaghi, and M. Abdolmaleki, Int. J. Res. Appl. Sci. Eng. Technol. 3, (2015).
R. K. Roy, A Primer on The Taguchi Method, 2nd ed. (Soc. Manuf. Eng., New York, 2010).
H. Atil and Y. Unver, Pakistan J. Biol. Sci. 3, 1538 (2000).
M. Edrissi, S. Samadanian-Isfahani, and M. Soleymani, Powder Technol. 249, 378 (2013).
J. Coates, Interpretation of Infrared Spectra, A Practical Approach, Encyclopedia of Analytical Chemistry (Wiley, Chichester, 2000).
C. Wu, X. Liu, D. Wei, J. Fan, and L. Wang, Water Res. 35, 3927 (2001).
M. Huang, C. Xu, Z. Wu, Y. Huang, J. Lin, and J. Wu, Dyes Pigm. 77, 327 (2008).
Y. Wang and C. Hong, Water Res. 33, 2031 (1999).
J. Saien, M. Asgari, A. R. Soleymani, and N. Taghavinia, Chem. Eng. J. 151, 295 (2009).
C. M. Zhu, L. Y. Wang, L. R. Kong, X. Yang, L. S. Wang, S. J. Zheng, et al., Chemosphere 41, 303 (2000).
C. M. So, M. Y. Cheng, J. C. Yu, and P. K. Wong, Chemosphere 46, 905 (2002).
M. Saquib and M. Muneer, Dyes Pigm. 56, 37 (2003).
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Reza Moradi, Mahdi Hamidvand & Amin Ganjali Using of TiO2/Ag2O Nanocomposite in Degradation of Acid Red 18 Dye in Photoreactor by Taguchi Experimental Design. Russ. J. Phys. Chem. 93, 1133–1142 (2019). https://doi.org/10.1134/S0036024419060268
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024419060268