Abstract
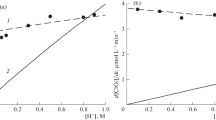
The effect of concentrations of Cl– and H+ ions, temperature, and ionic strength on the rate constant of the reaction between O3 and Cl–(aq) is studied experimentally. The reaction proceeds according to a mechanism of oxygen transfer and results in the formation of molecular chlorine in acidic medium. A formula is proposed for calculating the dependence of the rate constant on these factors.



Similar content being viewed by others
REFERENCES
A. V. Levanov, I. V. Kuskov, A. V. Zosimov, E. E. Antipenko, and V. V. Lunin, Kinet. Catal. 44, 740 (2003).
A. V. Levanov, E. E. Antipenko, and V. V. Lunin, Russ. J. Phys. Chem. A 86, 519 (2012).
A. V. Levanov, E. E. Antipenko, and V. V. Lunin, Russ. J. Phys. Chem. A 86, 2086 (2012).
A. V. Levanov, I. V. Kuskov, E. E. Antipenko, and V. V. Lunin, Russ. J. Phys. Chem. A 86, 757 (2012).
A. V. Levanov, I. V. Kuskov, E. E. Antipenko, and V. V. Lunin, Russ. J. Phys. Chem. A 82, 2045 (2008).
N. Kang, W. A. Jackson, P. K. Dasgupta, and T. A. Anderson, Sci. Total Environ. 405, 301 (2008).
K. J. Laidler, Pure Appl. Chem. 68, 149 (1996).
A. V. Levanov, O. Ya. Isaikina, R. V. Gasanova, and V. V. Lunin, Ind. Eng. Chem. Res. 57, 14355 (2018).
D. Maric, J. P. Burrows, R. Meller, and G. K. Moortgat, Photochem. Photobiol., A 70, 205 (1993).
S. P. Sander, J. Abbatt, J. R. Barker, et al., JPL Publ. No. 10-6 (Jet Propuls. Labor., Pasadena, 2011).
A. V. Levanov, I. V. Kuskov, A. V. Zosimov, E. E. Antipenko, and V. V. Lunin, J. Anal. Chem. 58, 439 (2003).
R. P. Bell, in Katalyse in Lösungen, Vol. 2 of Handbuch der Katalyse, Ed. by G.-M. Schwab (Springer, Wien, 1940), p. 191
G. M. Panchenkov and V. P. Lebedev, Chemical Kinetics and Catalysis, 3rd ed. (Khimiya, Moscow, 1985) [in Russian].
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by V. Avdeeva
Rights and permissions
About this article
Cite this article
Levanov, A.V., Isaikina, O.Y. & Lunin, V.V. Rate Constant of the Reaction between Ozone and Chloride Ion in an Aqueous Solution According to a Mechanism of Oxygen Atom Transfer. Russ. J. Phys. Chem. 93, 1045–1048 (2019). https://doi.org/10.1134/S0036024419060189
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024419060189