Abstract
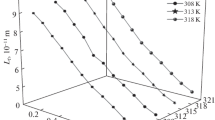
The ultrasonic velocity, density, and viscosity are measured at various concentrations 0.1–0.01 mol dm–3 in the solutions of 1,1-bis(4-(2-oxopropoxy)phenyl)cyclohexane (BMAPC) in 1,4-dioxane (DO), ethyl acetate (EA), tetrahydrofuan (THF) at 298–313 K and at atmospheric pressure. Parameters: acoustical impedance, adiabatic compressibility, Rao’s molar sound function, Van der Waals constant, internal pressure, free volume, intermolecular free path length, classical absorption coefficient and viscous relaxation time, Gibbs free energy of activation, enthalpy of activation and entropy of activation were determined. Change of the parameters with T indicated existence of strong molecular interactions in solutions and further supported by positive values of solvation number. Gibbs free energy of activation decrease linearly with increasing C and T in the DO system while it decreases with C and increase with T in EA and THF. Enthalpy and entropy of activation were found to be slightly concentration dependent.




Similar content being viewed by others
REFERENCES
R. K. Basal, J. Mittal, and P. Singh, J. Appl. Polym. Sci. 37, 1901 (1989).
B. F. Sels et al., Green Chem. 16, 1999 (2014).
H. K. Soni, V. S. Patel, and R. G. Patel, Thermochim. Acta 191, 307 (1991).
N. A. Karmishina, E. N. Rodlovskaya, B. A. Izmailov, V. A. Vasnev, and M. I. Buzin, Russ. J. Appl. Chem. 85, 674 (2012).
K. Sumoto, N. Mibu, K. Yokomizo, and M. Uyeda, Chem. Pharm. Bull. 52, 298 (2002).
R. Amorati, M. Lucarini, V. Mugnaini, and G. F. Pedulli, J. Org. Chem. 68, 5198 (2003).
A. Dhote and S. Aswale, Adv. Appl. Sci. Res. 3, 2299 (2012).
V. Frenkle, Adv. Drug. Deliv. Rev. 60, 1193 (2008).
D. R. Godhani, P. B. Dobariya, A. M. Sanghani, A. A. Jogel, and J. P. Mehta, J. Mol. Liq. 180, 179 (2013).
G. M. Kumar and S. A. Kumar, Russ. J. Chem. Sci. 3, 27 (2012).
B. R. Shinde and K. M. Jadhav, J. Eng. Res. Stud. 1, 128 (2010).
A. I. Vogel, A. R. Tatchell, B. S. Furnis, A. J. Hannaford, and P. W. D. Smith, Vogel’s Textbook of Practical Organic Chemistry, 5th ed. (Addison Wesley Longman, London, 1998).
B. B. Dhaduk, C. B. Patel, and P. H. Parsania, Lett. Drug. Des. Discov. 12, 152 (2015).
M. Habibullah, I. M. Rahman, M. A. Uddin, M. Anowar, M. Alam, K. Iwakabe, and H. Hasegawa, J. Chem. Eng. Data 58, 2887 (2013).
M. Gupta, I. Vibhu, and J. Shukla, Fluid Phase Equilib. 244, 26 (2006).
M. Gowrisankar, P. Venkateshwarlu, K. Sivakumar, and S. Sivarambabu, J. Solut. Chem. 42, 916 (2013).
H. Djojoputro and S. Ismadji, J. Chem. Eng. Data 50, 727 (2005).
B. B. Dhaduk, C. B. Patel, and P. H. Parsania, J. Solut. Chem. 44, 1976 (2015).
B. B. Dhaduk, C. B. Patel, and P. H. Parsania, Russ. J. Phys. Chem. A 91, 2495 (2017).
ACKNOWLEDGMENTS
The authors are thankful to UGC–New Delhi and DST–New Delhi for the instrumentation grants. Bhavin is also thankful to UGC–New Delhi for BSR Fellowship in Basic Science.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Dhaduk, B.B., Parsania, P.H. Molecular Interactions in Solutions of 1,1-Bis(4-(2-oxopropoxy)phenyl)cyclohexane on Ultrasonic Velocity, Density, and Viscosimetric Data. Russ. J. Phys. Chem. 93, 1065–1072 (2019). https://doi.org/10.1134/S0036024419060086
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024419060086