Abstract
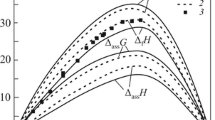
Thermochemical properties of liquid alloys of the Sn–Yb system are studied via calorimetry at 1110–1310 K over the ranges of concentrations 0 < xYb < 0.47 and 0.82 < xYb < 1. Considerable negative heat effects of the mixing of these alloys are identified. Activities of the components, the Gibbs energies and entropies of mixing, and the liquidus curve of the phase diagram of this system are calculated using the ideal associated solution (IAS) model and agree with data from the literature.
Similar content being viewed by others
References
X. J. Liu, M. H. Chen, Y. Lu, et al., CALPHAD 47, 129 (2014). doi 10.1016/j.calphad.2014.07.002
M. Mezbahul-Islam, A. O. Mostafa, and M. Medraj, J. Mater. 2014, 704283 (2014). doi 10.1155/2014/704283
K. A. Gschneidner, J. Less-Common Met. 17, 13 (1969).
W. C. M. Mattens, F. R. de Boer, A. K. Niessen, and A. R. Miedema, J. Magn. Magn. Mater. 31–34, 451 (1983).
A. Palenzona and S. Cirafici, J. Less-Common Met. 46, 321 (1976).
A. T. Dinsdale, Calphad 15, 319 (1991).
I. Barin and G. Platzki, Thermochemical Data of Pure Substances (VCH, Weinheim, New York, Basel, Cambridge, Tokyo, 1995).
CRC Handbook of Chemistry and Physics, 89th ed., Ed. by D. R. Lide (CRC, Taylor and Francis, Boca Raton, FL, 2009).
Binary Alloy Phase Diagrams, 2nd ed., Ed. by T. B. Masalsky (ASM International, Metals Park, OH, 1990).
M. V. Bulanova and V. R. Sidorko, IPMS Preprint (Frantsevich Inst. Probl. Mater. Sci. Natl. Acad. Sci. Ukrainy, Kiev, 1994).
A. Palenzona and S. Cirafici, J. Phase Equilib. 12, 482 (1991).
P. Manfrinetti, D. Mazzone, and A. Palenzona, J. Alloys Compd. 284, L1 (1999).
E. A. Leon-Escamilla and J. D. Corbett, Inorg. Chem. 38, 738 (1999).
E. A. Leon-Escamilla and J. D. Corbett, Inorg. Chem. 40, 1226 (2001).
J. N. Pratt and A. W. H. Morris, J. Less-Common Met. 10, 91 (1966).
C. Chatillon-Colinet, A. Percheron, J.-C. Mathieu, and J.-C. Achard, C. R. Acad. Sci. Paris 270, 473 (1970).
A. Palenzona, Thermochim. Acta 5, 473 (1973).
R. Boom, Scr. Metall. 8, 1277 (1974).
V. A. Lebedev, V. I. Kober, and L. F. Yamshchikov, Thermochemistry of Alloys of Rare-Earth and Actinide Elements: A Handbook (Metallurgiya, Chelyabinsk, 1989) [in Russian].
A. Yassin and R. Castanet, J. Alloys Compd. 307, 191 (2000).
V. T. Witusiewicz, V. R. Sidorko, and M. V. Bulanova, J. Alloys Compd. 248, 233 (1997).
M. Idbenali and C. Servant, J. Therm. Anal. Calorim. 103, 131 (2011). doi 10.1007/s10973-010-1082-4
M. Ivanov, V. Berezutski, and N. Usenko, J. Mater. Res. 102, 277 (2011).
V. G. Kudin, M. A. Shevchenko, I. V. Mateiko, and V. S. Sudavtsova, Zh. Fiz. Khim. 87, 364 (2013).
M. A. Shevchenko, M. I. Ivanov, V. V. Berezutskii, V. G. Kudin, and V. S. Sudavtsova, Russ. J. Phys. Chem. A 88, 897 (2014).
V. S. Sudavtsova, M. A. Shevchenko, V. V. Berezutskii, M. I. Ivanov, and V. G. Kudin, Russ. J. Phys. Chem. A 88, 200 (2014).
M. A. Shevchenko, V. G. Kudin, and V. S. Sudavtsova, Tr. IPM im. Frantsevicha NANU, Ser. Mod. Probl. Fiz. Materialoved. 21, 51 (2012).
G. Kaptay, Metall. Mater. Trans. A 43, 531 (2012).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © V.S. Sudavtsova, M.A. Shevchenko, M.I. Ivanov, A.S. Kozorezov, V.G. Kudin, 2018, published in Zhurnal Fizicheskoi Khimii, 2018, Vol. 92, No. 4, pp. 522–532.
Rights and permissions
About this article
Cite this article
Sudavtsova, V.S., Shevchenko, M.A., Ivanov, M.I. et al. Thermodynamic Properties of Alloys of the Sn–Yb System. Russ. J. Phys. Chem. 92, 630–639 (2018). https://doi.org/10.1134/S0036024418040295
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024418040295