Abstract
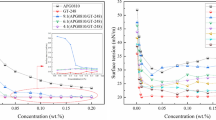
The physicochemical properties of initial formulation, that is anionic/amphoteric surfactants mixture SLES/AOS/CAB (sodium lauryl ether sulfate (SLES), α-olefin sulfonates (AOS) and cocamidopropyl betaine (CAB) at ratio 80 : 15 : 5) with nonionic surfactant of amine oxide type (lauramine oxide (AO)) in various concentration (1–5%) were studied. To characterize the surfactants mixture, the critical micelle concentration (CMC), surface tension (γ), foam volume, biodegradability and irritability were determined. This study showed that adding of AO in those mixtures lowered both γ and CMC as well as enhanced SLES/AOS/CAB foaming properties, but did not significantly affect biodegradability and irritability of initial formulation. Moreover, an increase in AO concentration has a meaningful synergistic effect on the initial formulation properties. All those results indicates that a nonionic surfactant of amine oxide type significantly improves the performance of anionic/amphoteric mixed micelle systems, and because of that anionic/amphoteric/nonionic mixture can be used in considerably lower concentrations as a cleaning formulation.
Similar content being viewed by others
References
J. Falbe, Surfactants in Consumer Products (Springer, Berlin, 1987).
J. Gambogi, S. Kennedy, and E. Ambundo, in Handbook of Detergents, Part E: Applications, Ed. by U. Zoller (Taylor and Francis Group, Boca Raton, FL, 2009).
M. J. Rosen, Surfactants and Interfacial Phenomenon, 3rd ed. (Wiley, New Jersey, 2004)
D. Meyers, Surfactant Science and Technology, 3rd ed. (VCH, New York, 2005)
T. Tamura, T. Iihara, S. Nishidaa, and S. Ohta, J. Surfact. Deterg. 2, 207 (1999).
T. P. Goloub, R. J. Pugh, and B. V. Zhmud, J. Colloid Interface Sci. 229, 72 (2000).
I. Bozetine, T. Ahmed Zaid, C. E. Chitour, and J. P. Canselier, J. Surfact. Deterg. 11, 299 (2008).
N. Jadidi, B. Adib, and F. B. Malihi, J. Surfact. Deterg. 16, 115 (2013).
S. N. Blagojević, S. M. Blagojević, and N. D. Pejić, J. Surfact. Deterg. 19, 363 (2016).
SRPS EN ISO 10707-2009. Water quality, Evaluation in an aqueous medium of the “ultimate” aerobic biodegradability of organic compounds, Method by analysis of biochemical oxygen demand (Closed bottle test) (Inst. Standardization of Serbia, 2009).
N. Grammer-West, J. E. Fitzpatrick, R. L. Jackson, H. Horton, and M. A. Damiano, J. Am. Acad. Dermatol. 35, 258 (1996).
M. Cronin, Report (John Moores Univ., Liverpool, 2005).
R. J. Williams, J. N. Phillips, and K. J. Mysels, Trans. Faraday Soc. 51, 728 (1995).
P. Carpena and J. Aguiar, P. Bernaola-Galván, and C. Carnero Ruiz, Langmuir 18, 6054 (2002).
C. Nitsch and G. Huttmann, SOFW J. 128 (5), 11 (2002).
Official Methods of Analysis of the Association of Official Analytical Chemists, Ed. by P. Cunniff, 16th ed. (AOAC, Arlington, 1995), Official Method 920.87.
Mild Surfactants for Personal Care Applications, Public ICC Personal Care, Clariant. http://www.essentialingredients. com/pdf/clariantmildsurfactants.pdf.
M. J. Rosen and B. Yao Zhu, J. Colloid Interface Sci. 99, 4 (1984).
M. J. Rosen and D. S. Murphy, J. Colloid Interface Sci. 110, 224 (1986).
A. Naved, K. A. Alamry, S. B. Khan, M. A. Rub, A. M. Asiri, and Y. Anwar, Int. J. Electrochem. Sci. 11, 1852 (2016).
S. Guernelli, A. Fontana, and R. Noto, D. Spinelli, and M. L. Turco Liveri, J. Colloid Interface Sci. 381, 67 (2012).
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Blagojević, S.M., Pejić, N.D. & Blagojević, S.N. Synergism and Physicochemical Properties of Anionic/Amphoteric Surfactant Mixtures with Nonionic Surfactant of Amine Oxide Type. Russ. J. Phys. Chem. 91, 2690–2695 (2017). https://doi.org/10.1134/S0036024417130064
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024417130064