Abstract
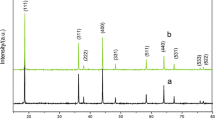
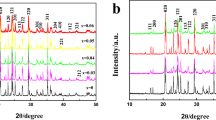
We report the synthesis of electrochemically active LiMn2O4 nanoparticles at varied temperature and pH values by sol–gel method using urea as a chelating and combusting agent. The effect of pH and annealing temperature on the structure, morphology and electrochemical performance was evaluated. The results obtained by XRD, SEM, TEM, and FTIR show that LiMn2O4 has uniform porous morphology and highly crystalline particles that can be obtained at pH 7.0 and 8.0 and at a relatively lower temperature of 600°C. Cyclic voltammetry measurements showed reversible redox reactions with fast kinetics corresponding to Li ions intercalation/deintercalation at 600°C at neutral pH 7.0. Charge/discharge studies carried out at a current rate of 40 mA g–1 reveal that LiMn2O4 synthesized at 600°C and pH 7.0 has the best structural stability and excellent cycling performance.
Similar content being viewed by others
References
F. F. Cao, Y. G. Guo, and L. J. Wan, Energy Environ. Sci. 4, 1634 (2011).
K. M. Shaju and P. G. Bruce, Chem. Mater. 20, 5557 (2008).
L. Tian and A. Yuan, J. Power Sources 192, 693 (2009).
M. Hirayama, H. Ido, K. S. Kim, W. Cho, K. Tamura, J. I. Mizuki, and R. Kanno, J. Am. Chem. Soc. 132, 15268 (2010).
B. J. Liddle, S. M. Collins, and B. M. Bartlett, Energy Environ. Sci. 3, 1339 (2010).
X. Li, F. Chang, B. Guo, and J. Chen, J. Phys. Chem. B 109, 14017 (2005).
D. Guan, J. A. Jeevarajan, and Y. Wang, Nanoscale 3, 1465 (2011).
J. Y. Luo, H. M. Xiong, and Y. Y. Xia, J. Phys. Chem. C 112, 12051 (2008).
Y. K. Sun and S. H. Jin, J. Mater. Chem. 8, 2399 (1998).
M. M. Thackeray, M. F. Mansuetto, D. W. Dees, and D. R. Vissers, Mater. Res. Bull. 31, 133 (1996).
M. H. Rossouw, A. de Kock, L. A. de Picciotto, M.M. Thackeray, W. I. F. David, and R. M. Ibberson, Mater. Res. Bull. 25, 173 (1990).
L. Pascual, H. Gadjov, D. Kovacheva, K. Petrov, P. Herrero, J. M. Amarilla, R. M. Rojas, and J. M. Rojo, J. Electrochem. Soc. 152, A301 (2005).
B. J. Hwang, R. Santhanam, and D. G. Liu, J. Power Sources 101, 86 (2001).
W. Tang, Y. Hou, F. Wang, L. Liu, Y. Wu, and K. Zhu, Nano Lett. 13, 2036 (2013).
Z. Kai, W. Yang, Z. Shuang, Y. Yan, P. Hao, L. Guiwei, and J. Jianli, Int. J. Electrochem. Sci. 9, 5280 (2014).
M. J. Iqbal and S. Zahoor, J. Power Sources 165, 393 (2007).
T. F. Yi, C. L. Hao, C. B. Yue, R. S. Zhu, and J. Shu, Synth. Met. 159, 1255 (2009).
M. J. Iqbal and R. A. Khan, J. Alloys Compd. 478, 847 (2009).
Y. S. Lee, Y. K. Sun, and K. S. Nahm, Solid State Ionics 109, 285 (1998).
R. Thirunakaran, A. Sivashanmugam, S. Gopukumar, and R. Rajalakshmi, J. Power Sources 187, 565 (2009).
R. Thirunakaran, A. Sivashanmugam, S. Gopukumar, Ch. W. Dunnill, and D. H. Gregory, Mater. Res. Bull. 43, 2119 (2008).
C. Z. Lu and G. T. K. Fey, J. Phys. Chem. Solids 67, 756 (2006).
Y. Gao, J. N. Reimers, and J. R. Dahn, Phys. Rev. B 54, 3878 (1996).
S. H. Ye, J. K. Bo, C. Z. Li, J. S. Cao, Q. L. Sun, and Y. L. Wang, Electrochim. Acta 55, 2972 (2010).
Y. Gao and J. R. Dahn, J. Electrochem. Soc. 143, 1783 (1996).
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Iqbal, A., Iqbal, Y., Khan, A.M. et al. Synthesis and Electrochemical Performance of Urea Assisted Pristine LiMn2O4 Cathode for Li Ion Batteries. Russ. J. Phys. Chem. 91, 2671–2679 (2017). https://doi.org/10.1134/S0036024417130040
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024417130040