Abstract
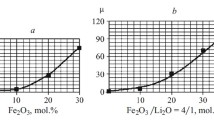
The glasses from the system xFe2O3(100 – x)[P2O5 · Li2О] with 0 < x < 50 mol % are studied. These materials have been prepared and characterized by magnetic susceptibility. Iron ions generally modify in different ways the local structure of these glasses, depending on the presence of Li2O in the glass matrix. The results have shown the presence of antiferromagnetic or ferromagnetic interactions between iron ions in the studied glass and temperature range. These data revealed that the valence states and the distribution of iron ions in the glass matrix depend on the Fe2O3 content, and can determine the decreasing of magnetic momentum (μeff).
Similar content being viewed by others
References
K. Srilatha, K. Sambasiva Rao, Y. Gandhi, V. Ravikumar, and N. Veeraiah, J. Alloys Compd. 507, 391 (2010).
T. Sankarappa, G. B. Devidas, M. P. Kumar, S. Kumar, and B. V. Kumar, J. Alloys Compd. 469, 576 (2009).
W. J. Chung, J. Choi, and Y. G. Choi, J. Alloys Compd. 505, 661 (2010).
S. P. Singh, R. P. S. Chakradhar, J. L. Rao, and B. Karmakar, Physica B 405, 2157 (2010).
A. Hirofumi, O. Satoshi, F. Koji, M. Shunsuke, and T. Katsuhisa, Phys. Rev. B 80, 134408 (2009).
P. Pascuta, G. Borodi, A. Popa, V. Dan, and E. Culea, Mater. Chem. Phys. 123, 767 (2010).
R. K. Singh and A. Srinivasan, J. Magn. Magn. Mater. 322, 2018 (2010).
S. Aqdim and M. Ouchetto, Adv. Mater. Phys. Chem. 3, 332 (2013).
R. K. Singh and A. Srinivasan, J. Magn. Magn. Mater. 322, 2018 (2010).
P. A. Bingham, R. J. Hand, S. D. Forder, and A. La Vay-sierre, J. Hazard. Mater. 119, 125 (2005).
R. K. Brow, J. Non-Cryst. Solids 1, 263 (2000).
R. Yang et al., J. Alloys Compd. 513, 97 (2012).
S. P. Chaudhuri and S. K. Patra, Mater. Sci. 35, 4735 (2000).
L. K. Wilson, E. J. Friebele, and D. L. Kinser, in Proceedings of the International Symposium on Amorphous Magnetism (Plenum, New York, 1975), p.65.
A. Mogus-Milankovic et al., Phys. Chem. Glasses 36, 31 (1995).
R. Iordanova et al., J. Non-Cryst. Solids 221, 227 (1998).
G. Srinivasarao and N. Veeraiah, J. Phys. Chem. Solids 63, 705 (2002).
C. Andronache, Mater. Chem. Phys. 136, 281 (2012).
B. Kumar and C. H. Chen, J. Appl. Phys. 75, 6760 (1994).
L. D. Bogomolova, N. A. Krasilnikova, V. L. Bogdanov, V. D. Khalilev, and V. Mitrofanov, J. Non-Cryst. Solids 188, 130 (1995).
A. M. Vorotinov and K. A. Sablina, Solid State Commun. 87, 209 (1993).
R. K. Singh, G. P. Kothiyal, and A. Srinivasan, J. Non-Cryst. Solids 354, 3166 (2008).
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Andronache, C., Balasoiu, M. & Racolta, D. Magnetic Interaction between Iron Particles in Lithium Phosphate Systems. Russ. J. Phys. Chem. 91, 2686–2689 (2017). https://doi.org/10.1134/S0036024417130039
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024417130039