Abstract
In this work, the synthesis of two asymmetric Co(II) and Ni(II) tetraazamacrocyclic complexes has been carried out by the template method. The synthesized complexes have been characterized by various analytical techniques such as elemental analysis, UV-Vis, IR, and Mass spectroscopy. The mass spectral studies of the complexes have been found to agree with theoretically calculated values. The calculated molar conductance values for the two complexes suggest a non-electrolytic nature of the complexes. A distorted octahedral geometry has been assigned to both the complexes as shown by spectral studies. Results of the electrochemical studies of the complexes through cyclic voltammetry indicate that metal centers can be differentiated by their intrinsic redox systems, viz. Co(III)/Co(II), Co(II)/Co(I) and Ni(II)/Ni(I). Moreover, antimicrobial activities of complexes have been investigated against E. coli, S. aureus, B. subtilis, and P. aeruginosa pathogens. The results of these studies indicate that both the complexes exhibit significant antimicrobial activity against the tested pathogens.
Similar content being viewed by others
INTRODUCTION
Macrocyclic complexes have been of considerable importance for their application in different sectors including photo-sensitization, solar-driven cells and electronics owing to their electron transport capabilities and the stabilization of uncommon oxidation states of the transition metal ion by macrocyclic ligand [1]. In recent decades, there observed the number of reports dedicated to the redox activity of transition metal macrocyclic complexes in non-aqueous systems have increased dramatically. This is particularly significant for those planned as models for the functional metalloprotein sites [2]. The redox potential of these substances (E1/2 for MLn+/ML(n + 1)+) is the main factor that not only represents the comparative thermodynamic stabilities of the oxidation states of central metals, and also defines the chemical reactivity of such complexes in redox systems [3, 4]. Recently, transition metals such as cobalt and nickel have been used for synthesis of complexes with diverse ligand and studied for their diverse properties [5–8]. In the early electrochemical studies of tetraazamacrocyclic complexes, the factors that influence the development of both oxidized and reduced metal species were identified [9–11]. It has been revealed that the overall redox activities of a particular system are determined by the following factor: (i) macrocyclic ring size, (ii) the ligand charge, (iii) the type of the ligand substituents, and (iv) degree of ligand unsaturation. The extent of ring strain inevitably affects the redox activity whereas a strong in-plane ligand field facilitates the oxidation attributable to the equatorial interactions [12, 13].
The macrocyclic structure offers an inherently planar environment for each central metal ion and facilitates coordinately unsaturated metal axial ligation. It can affect the macrocyclic complex’s redox behavior either by essentially changing the redox potential or by modifying the reduction site. Given that charged axial ligands usually occurred in extremely insoluble material, a selection of axial moieties can be done. Considering these facts, neutral ligands like N-donor heterocyclic compounds are utilized in this work. The redox activities of macrocyclic compounds may depend fairly on the axial ligation [14, 15].
Therefore, it is generally established that specific macrocyclic ligands have significant anti-bacterial, anti-herbicide, anti-insecticide, and anti-fungicide properties. The biological behaviors of bio-metals can be very often altered by the formation of chelates with specific bioligands. It is proposed that substances with antimicrobial efficacy can work either by destroying the bacterium or by suppressing the microbe’s multiplicity or preventing its active site. The antibacterial function of the macrocyclic complexes often relies, on the nature of the microbes.
Schiff’s bases macrocyclic ligand developed stable complexes with metal ions which play a key role in biochemical processes. The antibacterial agent in phagosomes and lysosomes destroys the consumed pathogen and enzymatically cleaves into smaller parts. In particular, respiratory disorders such as tuberculosis, acute respiratory tract infections, and severe acute respiratory syndrome pose a worldwide danger, and their occurrence is growing significantly over time. Even though there are several chemotherapy agents on the market, the bacterial species are increasing tolerance to such agents. Therefore, seeking better, more reliable, and cheaper pharmacologic agents is a big concern. Previously, comprehensive research on the development of macrocyclic complexes has evolved with particular respect to their antibacterial activity. Transition metal macrocyclic complexes were extensively examined for their antimicrobial and anticancer properties [16–19].
In this regard, we have synthesized asymmetric tetraazamacrocyclic complexes of Co(II) and Ni(II) by template method and characterized using multiple techniques. Further, both the macrocyclic complexes were examined for their antimicrobial activities against pathogens like E. coli, S. aureus, B. subtilis, and P. aeruginosa.
EXPERIMENTAL
Materials and methods. All the chemicals utilized in this study were of analytical grade. The chemicals triethylenetetraamine, 2,6-diacetyl pyridine, cobalt(II) chloride hexahydrate and nickel(II) chloride hexahydrate were procured from Merck, India. The chemicals were used with no further purification. Elemental analysis of synthesized materials was performed on Eager Xperience and TOF MS ES+6018e3 at CIL SAIF, Punjab University, Chandigarh. IR spectral analysis was performed on a Schimadzu 8400S spectrometer using KBr DRS system. UV-Vis spectra were obtained from a Schimadzu 2450 spectrophotometer. The conductance of synthesized complexes was evaluated using an Auto ranging Conductivity/TDS Meter (TCM+). The electrochemical measurements were carried out using a Cyclic Voltammeter of the Metrohm Model 663VA Stand potentiostat/galvanostat controlled by an external PC using the NOVA software and utilizing a three-electrode system at 25°C. A Pt disc (area 0.03 cm2) was used as working electrode which was polished with a H2O suspended Al2O3 before each trial. A Pt wire served as the counter electrode and Ag/AgCl (saturated KCl) was employed as the reference electrode. 0.1 M tetraethylammonium perchlorate (TEAP) was utilized as the supporting electrolyte.
Synthesis of macrocyclic complexes. The Schiff’s base tetraazamacrocyclic complexes {[M(L)X2]; M = Co(II), Ni(II), and X = C1−} were synthesized by following the reported method [20, 21]. Briefly, triethylenetetraamine (1 mol, 0.146 g), 2,6-diacetyl pyridine (1 mol, 0.163 g) and cobalt(II)chloride hexahydrate (1 mol, 0.237 g) were mixed in 50 mL MeOH in round bottomed flask. The reaction mixture was allowed to reflux for 7 h until a dark brown colored mixture was obtained. Lastly, the resulting mixture was concentrated using rotary evaporator and the concentrate was placed in desiccator overnight. A dark brown colored crystal of Co(II) complex was obtained which then recrystallized. In the same way, the macrocyclic complex of Ni(II) was also synthesized by using Ni(II) salt (0.238 g, 1 mol). The newly synthesized complexes were characterized by multiple techniques.
RESULTS AND DISCUSSION
The general scheme for the synthesis of two complexes is presented in Fig. 1. The synthesis of complexes was carried out using a ketone and an amine (consisting 4N-donor atoms) to get an asymmetric MN4-moiety. The thermodynamical stability of macrocyclic complexes is comparatively higher than open-chain analogs. Such facts have resulted into a broader interest to researchers. The complexes were synthesized through a simple synthetic route which prioritizes low reaction temperatures, better product yields and using easily available starting materials.
The synthesized complexes are colored and soluble in methanol, dimethylformamide, dichloromethane and acetonitrile solvents. Moreover, two complexes were found to be non hygroscopic and monomeric in nature and shown stability at room temperature. The molar conductance indicated a non-electrolytic behavior for the synthesized complexes. The physical and analytical data are presented in Table 1.
Electronic spectra of the Co(II) complex (Fig. 2a) is observed in DMSO (10–4 M) solution showed two weak intensity adsorption bands, 16 335 cm–1, (4T1g (F) → 4A2g), and 15246 cm–1 (4T1g (F) → 4A2g (F)). While, the electronic spectra of Ni(II) macrocyclic complex exhibit three absorption bands, 14233 cm–1 (3A2g (F) → 3T2g (F)), 15565 cm–1 (3A2g (F) → 3T1g(F)), 22 330 cm–1 (3A2g (F) → 3T1g (P)). These bands indicate that the complexes have distorted octahedral geometry [22].
IR spectral analysis was conducted for the key characterization about the formation of macrocyclic complexes. The appearance of moderate intensity bands in the range 3235–3250 cm–1 is caused by the N–H stretching in the complexes. The absorption bands in the range 1625–1628 cm–1 displayed the C=N stretching vibration that shows complete condensation of carbonyl and diamine. The moderate intensity bands in the 2845–2950 cm–1 region are caused by stretching vibration from C–H. The bands in the 465–480 cm–1 range are attributable to M–N stretching vibration, which also indicates formation of macrocyclic complexes. The IR spectra of the Co(II) complex is shown in Fig. 2b [23] and the data is given in Table 2.
Further, the mass spectra of the Ni(II) macrocyclic complex (Fig. 2c) displayed a molecular ion peak (M + 1) at m/z 401 and other peaks at m/z 301, 214, 200, and 178 could be assigned to the fragmentation pattern of macrocyclic complexes. Likewise, macrocyclic complex of Co(II) also exhibited the similar trend of fragmentations with a strong molecular ion peak [M + 1]+ at m/z 403 [24].
Cyclic Voltammetric (CV) Studies
Electrochemical studies of both complexes were performed using CV technique with DMSO consisting of TEAP (0.1M) as supporting electrolyte. The scan rate was maintained in the range of 50–200 mVs–1. The cyclic voltammogram (Fig. 3) of the Co(II) complex, recorded at 100 mVs–1 scan rate, exhibited two redox couples in the middle region of –0.7 V to +0.7 V. Based on peak separation, ∆E = 0.13–0.15 V and peak current ratio (ipa/ipc) that is close to unity, these redox process can be ascribed to quasirreversible process which can be assigned to Co(III)/Co(II), at higher potential, and Co(II)/Co(I), at lower potential. The CV plots of the Ni(II) complex (Fig. 3) were obtained under similar experimental conditions and one redox process corresponding to the Ni(II)/Ni(I) couple. This redox process was found to be quasirreversible with ∆E = 0.12 V and peak current ratio (ipa/ipc ≈ 1) [25, 26]. Further, the plots of ip against v1/2 were found to be linear for the Co(III)/Co(II) and Co(II)/Co(I) redox processes, indicating that these redox processes were regulated by diffusion, followed by the “Randles-Sevcik equation” for reversible electrochemical processes.
Biological Activity
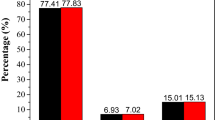
Further, both the complexes of Co(II) and Ni(II) were investigated for their antimicrobial activities by using agar well diffusion method against the E. coli, S. aureus, B. subtilis, P. aeruginosa and C. albicans pathogens. In this study, DMSO solvent and Gentamycin were used as negative and positive control media respectively and the antimicrobial activities were assessed by calculating the pathogens growth inhibition region. The Co(II) complex exhibited maximal inhibition zone versus E. coli (22 mm), whereas the complex of Ni(II) shows maximal inhibition zone versus S. aureus (20 mm). The antifungal activity of pathogen C. albicans, Ni(II) complex was observed to be more efficient (18 mm) than the Co(II) complex (17 mm) as data shown in Table 3.
CONCLUSIONS
In this report, two new asymmetric tetraazamacrocyclic complexes were prepared utilizing triethylenetetraamine, 2,6-diacetyl pyridine, and Co(II) and Ni(II) metal salts in a template process. The UV-Vis spectra analysis indicated an octahedral geometry for two macrocyclic complexes. The electrochemical studies were performed in the potential range –1.5 to +1.5 V vs Ag/AgCl at 100 mVs–1 scan rate for these complexes. The results demonstrated interesting features in stabilizing their uncommon oxidation states. For such macrocyclic structures heterogeneous electron transport rate constant and diffusion coefficient were also determined. The synthesized compounds demonstrated reasonable potential toward Gram +ve and Gram –ve microbes for antimicrobial activity, and were found to be comparable to the standard medication.
REFERENCES
A. N. Cain, T. N. C. Freeman, K. D. Roewe, et al., Dalton Trans. 48, 2785 (2019). https://doi.org/10.1039/C8DT04728F
N. Raman, J. D. Raja, and A. Sakthivel, J. Chil. Chem. Soc. 53, 1568 (2008). https://doi.org/10.4067/S0717-97072008000300003
A. Kumar and V. K. Vashistha, RSC Adv. 9, 13243 (2019). https://doi.org/10.1039/C9RA02169H
A. Kumar, V. K. Vashistha, P. Tevatia, and R. Singh, Anal. Bioanal. Electrochem. 3, 382 (2016). https://www.sid.ir/FileServer/JE/55002820160704
M. Salehi, M. Galini, M. Kubicki, and A. Khaleghian, Russ. J. Inorg. Chem. 64, 18 (2019). https://doi.org/10.1134/S0036023619010170
T. G. Cherkasova, N. V. Pervukhina, N. V. Kuratieva, et al., Russ. J. Inorg. Chem. 64, 1120, (2019). https://doi.org/10.1134/S0036023619090055
M. N. Zavalishin, G. A. Gamov, A. Y. Khokhlova, et al., Russ. J. Inorg. Chem. 65, 119 (2020). https://doi.org/10.1134/S0036023620010209
A. S. Pronin, S. A. Semenov, D. V. Drobot, and G. I. Dzhardimalieva, Russ. J. Inorg. Chem. 63, 1041, (2018). https://doi.org/10.1134/S0036023618080193
S. H. Sumrra, and H. C. Zahid, Spectrochim. Acta A 98, 53 (2012). https://doi.org/10.1016/j.saa.2012.08.026
M. Gaber, E.-G. Hoda, F. Atlam, and S. Fathalla, Spectrochim. Acta A 137, 919 (2015). https://doi.org/10.1016/j.saa.2014.09.015
V. K. Vashistha and A. Kumar, Inorg. Chem. Commun. 112 (2020) 107700. https://doi.org/10.14233/ajchem.2019.21952
S. H. Sumrra, M. Ibrahim, S. Ambreen, et al., Bioinorg. Chem. Appl. (2014). https://doi.org/10.1155/2014/812924
S. Koch, R. H. Holm, and R. B. Frankel, J. Am. Chem. Soc. 97, 6714 (1975). https://doi.org/10.1021/ja00856a020
V. K. Vashistha, Y. Kumar, A. Kumar, and D.K. Das, 78, 788, (2019). http://nopr.niscair.res.in/handle/123456789/51188.
W. Grochala, A. Jagielska, K. Woźniak, et al. Fauci. Nature, 430, 242 (2004). https://www.nature.com/articles/nature02759.
D. M. Skowronski, C. Astell, R. C. Brunham, et al., Annu. Rev. Med. 56, 357 (2005).
V. K. Vashistha, N. Sharma, A. Kumar, and U. Sharma, Asian J. Chem. 31, 2116 (2019). https://doi.org/10.14233/ajchem.2019.21952
A. Kumar, V.K. Vashistha, P. Tevatia, and R. Singh, Spectrochim. Acta A 176, 123 (2017). https://doi.org/10.1016/j.saa.2016.12.011
Sweety, V. K. Vashistha, A. Kumar, and R. Singh, Russ. J. Electrochem. 55, 161 (2019). https://doi.org/10.1134/S1023193519020113
A. Kumar, V. K. Vashistha, P. Tevatia, and R. Singh, Spectrochim. Acta A 176, 123 (2017). https://doi.org/10.1016/j.saa.2016.12.011
F. L. Lindoy, The Chemistry of Macrocyclic Ligand Complexes (Cambridge Univ. Press, 1990).
V. K. Vashistha, D. K. Das, A. Yadav, D. Saini, and A. Kumar, Anal. Bioanal. Electrochem. 12, 318 (2020). http://www.abechem.com/article_38852.html.
X. Yu, and J. Zhang. Macrocyclic Polyamines: Synthesis and Applications (Wiley, 2017).
E. Franco, E. Lopez-Torres, M. Mendiola, and M. Sevilla, Polyhedron 19, 441 (2000). https://doi.org/10.1016/S0277-5387(99)00383-6
S. Chandra, N. Gupta, and R. Gupta, Spectrochim. Acta A 63, 587, (2006). https://doi.org/10.1016/j.saa.2005.06.005
Funding
The authors are thankful to GLA University Mathura for the financial support and support is also acknowledged for completion the studies from SAIF Panjab University Chandigarh.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Authors have no conflict of interest to declare.
Rights and permissions
About this article
Cite this article
Vashistha, V.K., Kumar, A. Synthesis of Co(II) and Ni(II) Asymmetric Tetraazamacrocyclic Complexes and Their Electrochemical and Antimicrobial Studies. Russ. J. Inorg. Chem. 65, 2028–2032 (2020). https://doi.org/10.1134/S0036023620140077
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023620140077