Abstract
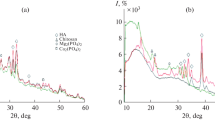
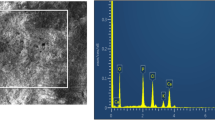
We present the results of characterization of prepared composites comprising dicalcium phosphate dihydrate (DCPD, brushite) and hydroxyapatite in the polymeric matrix of chitin and chitosan. The crystallite sizes increase as the chitosan and chitin percentage in the composite increases. When samples are dissolved in isotonic saline, the degradation rate of brushite composites decreases as the chitosan and chitin percentage in the sample increases; for hydroxyapatite composites, an opposite trend is typical. The samples are shown to change their weight as a result of heat treatment.








Similar content being viewed by others
REFERENCES
K. L. Kandyrin, Introduction to the Materials Science of Polymers (IPTs MITKhT, Moscow, 2002) [in Russian].
A. Yu. Khomenko, P. V. Popryadukhin, and T. B. Bogomolova, Ross. Nanotekhnol. 8, 41 (2013).
L. A. Nud’ga, Doctoral Dissertation in Chemistry (2006).
R. Jayakumar, K. Chennazhi, and S. Srinivasan, Int. J. Mol. Sci., No. 12, 1876 (2011).
T. G. Volova, Materials for Medicine, Cell and Tissue Engineering (IPK SFU, Krasnoyarsk, 2009) [in Russian].
S. N. Danil’chenko, O. V. Kalinkevich, and M. V. Pogorelov, Ortoped., Travmatol. Protez., No. 1, 66 (2009).
V. E. Kamskaya, Biol. Nauki, No. 6, 36 (2016).
E. S. de Alvarenga, Univ. Fed. Viçosa, 91 (2011).
V. P. Kurchenko, S. V. Buga, N. V. Petrashkevich, et al., Tr. BGU, 11 (2016).
A. A. Murav’ev, Candidate’s Dissertation in Chemistry (Moscow, 2017).
D. A. Wahl, Eur. Cells Mater., No. 11, 43 (2006).
A. O. Bolarinwa, School Chem. Eng., 220 (2010).
Cuneyt A. Tas, J. Am. Ceram. Soc., 1200 (2016).
B. B. Parekh and M. J. Joshi, J. Pure Appl. Phys. 43, 675 (2005).
S. V. Dorozhkin, Materials, No. 2, 399 (2009).
V. B. Suryawanshi and R. T. Chaudhari, J. Mater. Sci., 6 (2014).
S. Gurbhinder, J. Miner. Mater. Charact. Eng. 10, 727 (2011).
N. V. Petrakova, Candidate’s Dissertation in Technical Science (Moscow, 2014).
A. V. Knyazev and E. N. Bulanov, Vestn. Nizhegorodsk. Univ. Im. N.I. Lobachevskogo 5 (1), 88 (2012).
N. V. Bakunova, S. M. Barinov, V. S. Komlev, et al., Nauchn. Ved., Ser. Mat., No. 11 (106), 173 (2011).
A. N. Gurin, Candidate’s Dissertation in Medical Science (Moscow, 2009).
T. V. Safronova, P. A. Seichenko, and V. I. Putlyaev, Steklo Keram., No. 8, 34 (2012).
R. R. Izmailov, O. A. Golovanova, Y. V. Tserikh, et al., Russ. J. Inorg. Chem. 61, 817 (2016). https://doi.org/10.1134/S0036023616070081
S. Sagadevan, J. Phys. Sci. 8, 1639 (2013).
A. P. Solonenko and O. A. Golovanova, Russ. J. Inorg. Chem. 58, 1420 (2013). https://doi.org/10.1134/S0036023614010173
L. Jiang, Carbohydr. Polym. 74, 680 (2008).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Translated by O. Fedorova
Rights and permissions
About this article
Cite this article
Fadeeva, T.V., Golovanova, O.A. Physicochemical Properties of Brushite and Hydroxyapatite Prepared in the Presence of Chitin and Chitosan. Russ. J. Inorg. Chem. 64, 847–856 (2019). https://doi.org/10.1134/S0036023619070064
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023619070064