Abstract
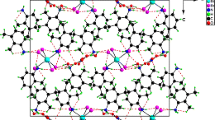
The structure of (C3H6N3)4Bi2Cl10 was determined by single crystal X-ray diffraction at room temperature. It crystallizes in the orthorhombic space group Pcmn, with a = 9.430 (1) Å, b = 17.426 (3) Å, c = 19.883(5) Å, V = 3267.3 (11) Å3 and Z = 4. The structure consists of discrete binuclear [Bi2Cl10]4– anions and 3-aminopyrazolium cations. The crystal packing is governed by weak N–H···Cl hydrogen bonds, π–π and electrostatic Cl···Cl interactions. Infrared spectrum is used to gain more information on the title compound. An assignment of the observed vibration modes is reported. The crystal morphology is studied using the BFDH laws. The calculated HOMO and LUMO energies show that charge transfer occur within organic and inorganic molecules. The optical absorption of the zero-dimensional hybrid was also investigated.
Similar content being viewed by others
References
J. Lü, E. H. Shen, Y. G. Li, et al., Cryst. Growth & Des. 5, 65 (2005).
D.B. Mitzi and P. Brock, Inorg. Chem. 40, 2096 (2001).
H. Ferjani, H. Boughzala, and A. Driss, Acta Crystallogr. Sect. E 68, m615 (2012).
M. A. Tershansy, A. M. Goforth, M. D. Smith, et al., Acta Crystallogr. Sect. E 62, m3269 (2006).
A. Piecha, R. Jakubas, G. Bator, and J. Baran, Vib. Spectrosc. 51, 226 (2009).
T. Ben Rhayem, H. Boughzala, A. Driss, Acta Crystallogr. Sect. E 69, m330 (2013).
D. B. Mitzi, C. D. Dimitrakopoulos, J. Rosner, et al., Adv. Mater. 14, 1772 (2002).
D. B. Mitzi, K. Chondroudis, and C. R. Kagan, IBM. J. Res. Dev. 45 (1), 29 (2001).
W. Bi, N. Louvain, N. Mercier, et al., Adv. Mater. 20, 1013 (2008).
A. M. Goforth, L. Peterson, Jr., M. D. Smith., and H.-C. Z. Loye., J. Solid State Chem. 178, 3529 (2005).
G. C. Papavassiliou, G. A. Mousdis, C. P. Raptopoulou, and A. Terzis, Z. Naturforsch., B: Chem. Sci. 54, 1405 (1999).
C. Feldmann, J. Solid State Chem. 172, 53 (2003).
H. Krautscheild and F. Vielsack, J. Chem. Soc., Dalton Trans. 16, 2731 (1999).
G. A. Fisher and N. C. Norman, Adv. Inorg. Chem. 41, 233 (1994).
A. J. M. Duisenberg, J. Appl. Crystallogr. 25, 92 (1992).
A. C. T. North, D. C. Phillips, and F. S. Mathews, Acta Crystallogr., Sect. A: Found. Crystallogr. 24, 351 (1968).
G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr. 64, 112 (2008).
K. Brandenburg, DIAMOND, Crystal Impact (GbR, Bonn, Germany, 2008).
Mercury CSD 3.0.1 (Build RC6) (Cambridge Crystallographic Data Centre (CCDC), 2013).
A. Coelho, Topas V 4.2 (Bruker AXS, 2007–2009).
J. P. H. Charmant, N. C. Norman, J. Starbuck, Acta Crystallogr. Sect. E 58, m144 (2002).
G. A. Bowmaker, P. C. Junk, A. M. Lee, et al., Aust. J. Chem. 51, 293 (1998).
A. Bravais, Etudes Cristallographiques (Gauthier-Villars, Paris, France, 1913).
G. Fridel, Bull. Soc. Fr. 30, 326 (1907).
J. D. H. Donnay and D. Harker, Am. Mineral. 22, 446 (1937).
V. M. Leovac, Z. D. Tomić, A. Kovács, et al., J. Organomet. Chem. 693, 77 (2008).
J. A. Jimenez, R. M. Claramunt, O. Mó, et al., Phys. Chem. Chem. Phys. 1, 5113 (1999).
E. Kavitha, N. Sundaranganesan, and S. Sebastian, Ind. J. Pure Appl. Phys. 48, 20 (2010).
O. Prasad, L. Sinha, and N. Kumar, J. At. Mol. Sci. 1, 201 (2010).
I. Fleming, Frontier Orbitals and Organic Chemical Reactions (Wiley, New York, 1976).
Cache: Work System Pro Version 7.5.0.85 (Fujitsu Limited. 2000–2006 Oxford Molecular).
D. F. V. Lewis, C. Ioannides, and D. V. Parke, Xenobiotica. 24, 401 (1994).
K. Oldenburg, A. Vogler, I. Mikό, and O. Horváth, Inorg. Chim. Acta 248, 107 (1996).
A. Vogler, A. Paukner, and H. Kunkely, Coord. Chem. Rev. 97, 285 (1990).
A. Vogler and H. Nikol, Pure Appl. Chem. 64, 1311 (1992).
H. Nikol and A. Vogler, J. Amer. Chem. Soc. 113, 8986 (1991).
W. R. Mason, Inorg. Chem. 38, 2742 (1999).
M. V. S. Prasad, K. Chaitanya, N. U. Sri, and V. Veeraiah, Spectrochim. Acta, Pt A: Mol. Biomol. Spectrosc. 99, 379 (2012).
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Ferjani, H., Boughzala, H. New Hybrid Material: (C3H6N3)4Bi2Cl10. Synthesis, Structural Study and Spectroscopic Behavior. Russ. J. Inorg. Chem. 63, 349–356 (2018). https://doi.org/10.1134/S0036023618030099
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023618030099