Abstract
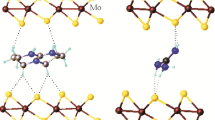
The atomic structure of the layered nanocrystalline molybdenum disulfide compound with trimethylphenylammonium cations has been determined for the first time using X-ray powder diffraction analysis adapted for turbostratically disordered systems and quantum chemical density functional theory calculations. It has been demonstrated that, in this compound, a conducting modification of MoS2 monolayers is stabilized, which is metastable under common conditions. Bonding interaction inside the molybdenum disulfide layers as well as between these layers and organic cations, revealed in the framework of Bader’s atoms in molecules theory, has been considered.
Similar content being viewed by others
References
C. T. Tye and K. J. Smith, Top. Catal. 37, 129 (2006).
J.-F. Yang, B. Parakash, J. Hardell, and Q.-F. Fang, Front. Mater. Sci. 6, 116 (2012).
B. Radisavljevic, A. Radenovic, J. Brivio, et al., Nat. Nanotechnol. 6, 147 (2011).
A. Nourbakhsh, A. Zubair, M. S. Dresselhaus, and T. Palacios, Nano Lett. 16, 1359 (2016).
J. D. Benck, T. R. Hellstern, J. Kibsgaard, et al., ACS Catal. 4, 3957 (2014).
X. Zong, G. Wu, H. Yan, et al., J. Phys. Chem. C 114, 1963 (2010).
T. Jia, M. M. J. Li, L. Ye, et al., Chem. Commun. 51, 13496 (2015).
M. Acerce, D. Voiry, and M. Chhowalla, Nat. Nanotechnol. 10, 313 (2015).
M. A. Py and R. R. Haering, Can. J. Phys. 61, 76 (1983).
A. N. Enyashin and G. Seifert, Comp. Theor. Chem. 999, 13 (2012).
R. Lv, J. A. Robinson, R. E. Schaak, et al., Acc. Chem. Res. 48, 56 (2015).
F. Wypych and R. Schollhorn, J. Chem. Soc., Chem. Commun., No. 19, 1386 (1992).
A. S. Goloveshkin, I. S. Bushmarinov, N. D. Lenenko, et al., J. Phys. Chem. C 117, 8509 (2013).
A. S. Golub, Y. V. Zubavichus, Y. L. Slovokhotov, et al., Solid State Ion. 128, 151 (2000).
P. Joensen, R. F. Frindt, and S. R. Morrison, Mater. Res. Bull. 21, 457 (1986).
A. S. Goloveshkin, N. D. Lenenko, V. I. Zaikovskii, et al., RSC Adv. 5, 19206 (2015).
A. S. Goloveshkin, I. S. Bushmarinov, A. A. Korlyukov, et al., Langmuir 31, 8953 (2015).
TOPAS 5.0 User Manual, Bruker AXS GmbH, Karlsruhe, Germany, 2014.
G. Kresse and J. Furthmuller, Comput. Mater. Sci. 6, 15 (1996).
G. Kresse and J. Furthmuller, Phys. Rev. B 54, 11169 (1994).
S. Grimme, J. Comput. Chem. 27, 1787 (2006).
G. Kresse and D. Joubert, Phys. Rev. 59, 1758.
X. Gonze, J.-M. Beuken, R. Caracas, et al., Comp. Mater. Sci. 25, 478 (2002).
R. Bader, Atoms in Molecules: A Quantum Theory, (Clarendon, 1994).
W. Tang, E. Sanville, and G. Henkelman, J. Phys.: Condens. Matter 21, 84204 (2009).
K. Ufer, G. Roth, R. Kleeberg, et al., Z. Kristallogr. 219, 519 (2004).
R. A. Gordon, D. Yang, E. D. Crozier, et al., Phys. Rev. B 65, 125407 (2002).
X. Wang, J. Li, R. D. Hart, et al., J. Appl. Crystallogr. 44, 902 (2011).
A. V. Powell, L. Kosidowski, and A. McDowall, J. Mater. Chem. 11, 1086 (2001).
F. Allen, Acta Crystallogr., Sect. B 58, 380 (2002).
J. Van De Streek and M. A. Neumann, Acta Crystallogr., Sect. B 66, 544 (2010).
E. Espinosa, E. Molins, and C. Lecomte, Chem. Phys. Lett. 285, 170 (1998).
E. Espinosa, C. Lecomte, and E. Molins, Chem. Phys. Lett. 300, 745 (1999).
N. G. Naumov, A. A. Korlyukov, D. A. Piryazev, et al., Izv. Akad. Nauk, Ser. Khim., No. 8, 1852 (2013).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © A.S. Goloveshkin, I.S. Bushmarinov, A.A. Korlyukov, N.D. Lenenko, A.S. Golub’, I.L. Eremenko, 2017, published in Zhurnal Neorganicheskoi Khimii, 2017, Vol. 62, No. 6, pp. 743–750.
Rights and permissions
About this article
Cite this article
Goloveshkin, A.S., Bushmarinov, I.S., Korlyukov, A.A. et al. Atomic structure and bonding interaction in a layered molybdenum disulfide compound with trimethylphenylammonium cations. Russ. J. Inorg. Chem. 62, 729–735 (2017). https://doi.org/10.1134/S0036023617060080
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023617060080