Abstract
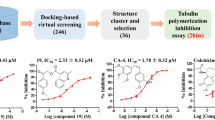
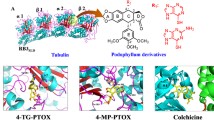
Cytostatic colchicine is widely used in the treatment of Familial Mediterranean fever, but it has several side effects. For finding new, more effective drugs with higher affinity and diminishside effects we carried out virtual screening of potential inhibitors of the main target of colchicine, the polymerization of tubulin by evaluating affinity 25745 compounds, structurally related to the colchicine. We have identified 11 commercially available compounds with higher affinity to tubulin. Compounds with highest binding scores include trimethoxybenzene and its derivatives; these compounds bind to the same site in similar orientation. Information provided can form the basis for design of new cytostatics.
Similar content being viewed by others
Abbreviations
- CBS:
-
Colchicine Binding Site
- CID:
-
Compound Identifier
- FMF:
-
Familial Mediterranean Fever
- ICM:
-
Internal Coordinates Mechanics
- MAPs:
-
Microtubule-Associated Proteins
- SAR:
-
Structure-Activity Relationships
References
Coakley W.T. 1987. Hyperthermia effects on the cytoskeleton and on cell morphology. Symp. Soc. Exp. Biol. 41, 187–211.
Menéndez M., Rivas G., Díaz J.F., Andreu J.M. 1998. Control of the structural stability of the tubulin dimer by one high affinity bound magnesium ion at nucleotide N-site. J. Biol. Chem. 273 (1), 167–176.
Zhang R., Alushin G.M., Brown A., Nogales E. 2015. Mechanistic origin of microtubule dynamic instability and its modulation by EBproteins. Cell. 162 (4), 849–859.
Weisenberg R.C., Broisy G.G., Taylor E.W. 1968. Colchicine-binding protein of mammalian brain and its relation to microtubules. Biochemistry. 7 (12), 4466–4479.
Panda D., Daijo J.E., Jordan M.A., Wilson L. 1995. Kinetic stabilization of microtubule dynamics at steady state in vitro by substoichiometric concentrations of tubulin-colchicine complex. Biochemistry. 34 (31), 9921–9929.
Prota A.E., Bargsten K., Zurwerra D., Field J.J., Díaz J.F., Altmann K.H., Steinmetz M.O. 2013. Molecular mechanism of action of microtubule-stabilizing anticancer agents. Science. 339 (6119), 587–590.
Jordan M.A., Wilson L. 2004. Microtubules as a target for anticancer drugs. Nat. Rev. Cancer. 4 (4), 253–265.
Lu Y., Chen J., Xiao M., Li W., Miller D.D. 2012. An overview of tubulin inhibitors that interact with the colchicine binding site. Pharm. Res. 29 (11), 2943–2971.
Ravelli R.B., Gigant B., Curmi P.A., Jourdain I., Lachkar S., Sobel A., Knossow M. 2004. Insight into tubulin regulation from a complex with colchicine and a stathmin-like domain. Nature. 428 (6979), 198–202.
Liu W., Sun P., Yang L., Wang J., Li L., Wang J. 2010. Assay of glioma cell responses to an anticancer drug in a cell-based microfluidic device. Microfluidics Nanofluidics. 9 (4–5), 717–725.
Cho J.H., Joo Y.H., Shin E.Y., Park E.J., Kim M.S. 2017. Anticancer effects of colchicine on hypopharyngeal cancer. Anticancer Res. 37 (11), 6269–6280.
Lidar M., Livneh A. 2007. Familial Mediterranean fever: Clinical, molecular and management advancements. Neth. J. Med. 65 (9), 318–324.
Emmerson B.T. 1996. The management of gout. New Engl. J. Med. 334 (7), 445–451.
Deursen R.V., Blum L.C., Reymond J.L. 2010. A searchable map of PubChem. J. Chem. Inform. Modeling. 50 (11), 1924–1934.
Prota A.E., Danel F., Bachmann F., Bargsten K., Buey R.M., Pohlmann J., Reinelt S., Lane H., Steinmetz M.O. 2014. The novel microtubule-destabilizing drug BAL27862 binds to the colchicine site of tubulin with distinct effects on microtubule organization. J. Mol. Biol. 426 (8), 1848–1860.
Marangon J., Christodoulou M.S., Casagrande F.V., Tiana G., Dalla Via L., Aliverti A., Passarella D., Cappelletti G., Ricagno S. 2016. Tools for the rational design of bivalent microtubule-targeting drugs. Biochem. Biophys. Res. Commun. 479 (1), 48–53.
Gaspari R., Prota A. E., Bargsten K., Cavalli A., Steinmetz M.O. 2017. Structural basis of cis-and trans-combretastatin binding to tubulin. Chem. 2 (1), 102–113.
Bottegoni G., Kufareva I., Totrov M., Abagyan R. 2009. Four-dimensional docking: A fast and accurate account of discrete receptor flexibility in ligand docking. J. Med. Chem. 52 (2), 397–406.
Totrov M. 2011. Ligand binding site superposition and comparison based on atomic property fields: Identification of distant homologues, convergent evolution and PDB-wide clustering of binding sites. BMC Bioinformatics. 12 (1), 35.
Sadovnichy V., Tikhonravov A., Voevodin V., Opanasenko V. 2013. “Lomonosov”: Supercomputing at Moscow State University. Contemporary High Performance Computing: From Petascale toward Exascale. Chapman & Hall/CRC Computational Science, Boca Raton, FL: CRC Press, pp. 283–307.
Kaur R., Kaur G., Gill R.K., Soni R., Bariwal J. 2014. Recent developments in tubulin polymerization inhibitors: An overview. Eur. J. Med. Chem. 87, 89–124.
Olazarán F.E., García-Pérez C.A., Bandyopadhyay D., Balderas-Rentería I., Reyes-Figueroa A.D., Henschke L., Rivera G. 2017. Theoretical and experimental study of polycyclic aromatic compounds as β-tubulin inhibitors. J. Mol. Model. 23 (3), 85.
Nogales E., Wolf S.G., Downing K.H. 1998. Structure of the αβ tubulin dimer by electron crystallography. Nature. 391 (6663), 199–203.
Varzhabetyan L.R., Glazachev D.V., Nazaryan K.B. 2012. Molecular dynamics simulation study of tubulin dimer interaction with cytostatics. Mol. Biol. (Moscow). 46 (2), 316–321.
Nepali K., Ojha R., Sharma S., Bedi P., Dhar K. 2014. Tubulin inhibitors: A patent survey. Recent Pat. Anticancer Drug Discov. 9 (2), 176–220.
Chaudhary V., Venghateri J.B., Dhaked H.P., Bhoyar A.S., Guchhait S.K., Panda D. 2016. Novel combretastatin-2-aminoimidazole analogues as potent tubulin assembly inhibitors: Exploration of unique pharmacophoric impact of bridging skeleton and aryl moiety. J. Med. Chem. 59 (7), 3439–3451.
Salum L.B., Altei W.F., Chiaradia L.D., Cordeiro M.N., Canevarolo R.R., Melo C.P., Winter E., Mattei B., Daghestani H.N., Santos-Silva M.C., Creczynski-Pasa T.B., Yunes R.A., Yunes J.A., Andricopulo A.D., Day B.W., et al. 2013. Cytotoxic3,4,5-trimethoxychalcones as mitotic arresters and cell migration inhibitors. Eur. J. Med. Chem. 63, 501–510.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Molekulyarnaya Biologiya, 2018, Vol. 52, No. 4, pp. 699–704.
The article is published in the original.
Rights and permissions
About this article
Cite this article
Sahakyan, H.K., Arakelov, G.G. & Nazaryan, K.B. In silico Search for Tubulin Polymerization Inhibitors. Mol Biol 52, 604–608 (2018). https://doi.org/10.1134/S0026893318040179
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0026893318040179