Abstract
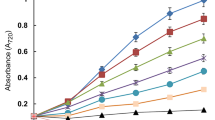
Arsenite (As(III)) induced changes were investigated in the diazotrophic cyanobacterium Anabaena PCC 7120. The cells of Anabaena sp. strain PCC 7120 exposed to As(III) produced H2O2 and its production increased with increase in As(III) concentration. As a part of resistant mechanism and also to counteract the deleterious effect of H2O2, the cells of Anabaena sp. strain PCC 7120 produced more ascorbate peroxidase (APx) whose level also increased in response to As(III) concentrations. The increase in APx activity was directly proportional to the increase in H2O2 production and maximum APx activity was recorded at 40 ppm of As(III). In contrast, glutathione reductase (GR) activity decreases with increase in As(III) concentrations and attained its minimum level at 40 ppm of As(III). Lipid peroxidation increased with increase in As(III) concentration and maximum peroxidation (which was about two fold higher than that of the untreated control cells) was recorded at 40 ppm of As(III). Exposure of the Anabaena sp. strain PCC 7120cells to As(III) has also resulted in a significant increase in ascorbate and dehydro-ascorbate contents which was about 6.44 and 1.97 fold higher than that of the untreated control cells, respectively at 40 ppm of As(III).











Similar content being viewed by others
Notes
Abbreviations: AAS—Atomic absorption spectrophotometer, APx—Ascorbate peroxidase, As(III)—Arsenite, As(V)—Arsenate, As—Arsenic, ASA—Ascorbic acid, Asc—Ascorbate, CAT—Catalase, Chl a—Chlorophyll a, DAB—3,3'-diaminobenzidine, DAs—Dehydro-ascorbate, DTT—Dithiothreitol, EDTA—Ethylene diamine tetraacetic acid, GPx—Guaiacol peroxidise, GR—Glutathione reductase, GSH—Glutathione, H2O2—Hydroperoxides, LC—Lethal concentration, MDA—Malondialdehyde, NADPH—Nicotinamide adenine dinucleotide phosphate, NBT—Nitro Blue tetrazolium, PUFA—Poly unsaturated Fatty acids, ppm—parts per million, PVP—Polyvinyl pyrrolidone, ROS—Reactive oxygen species, SH—sufhydryl, SOD—Superoxide dismutase, TBA—Thiobarbituric acid, TCA—Trichloroacetic acid.
REFERENCES
Aebi, H. Catalase, in Methods in Enzymology, Packer, L., Ed., Orlando: Academic, 1984, vol. 105, pp. 121–126.
Bartosz, G., Oxidative stress in plants, Acta Physiol., Plant., 1997, vol. 19, pp. 47–64.
Bhattacharjee, H., Mukhopadhyay, R., Thiyagarajan, S., and Rosen, B.P., Aquaglyceroporin: ancient channel for metalloids, J. Biol., 2008. vol. 7, pp. 33–35.
Bhattacharya, P. and Pal, R., Response of cyanobacteria to arsenic toxicity, J. Appl. Phycol., 2011, vol. 23, pp. 293–299.
Castillo, F.I., Penel, I., and Greppin, H., Peroxidase release induced by ozone in Sedum album leaves, Plant Physiol., 1984, vol. 74, pp. 846–851.
Cerutti, P.A., Prooxidant states and tumor promotion, Science, 1985, vol. 227, pp. 375–381.
Gebel, T. Confounding variables in the environmental toxicology of arsenic, Toxicol., 2000, vol. 144, pp. 155–162.
Giannopolitis, C. and Ries, N. Superoxide dismutase I: occurrence in higher plants, Plant Physiol., 1977, vol. 59, pp. 309–314.
Gratão, P.L., Polle, A., Lea, P.J., and Azevedo, R.A., Making the life of heavy metal-stress plants a little easier, Funct. Plant Biol., 2005, vol. 32, pp. 481–494.
Hepler, P.K. and Wayne, R.O., Calcium and plant development, Annu. Rev. Plant Physiol., 1985, vol. 36, pp. 397–439.
Hussein, K.A. and Joo, J.H., Heavy metal resistance of bacteria and its impact on the production of antioxidant enzymes, African J. Microbiol. Res., 2013, vol. 7, no. 20, pp. 288–2296.
Kenney, L. and Kaplan, J.H., Arsenate substitutes for phosphate in the human red cell sodium pump and anion exchanger, J. Biol. Chem., 1988, vol. 263, pp. 7954–7960.
Kwon, S.I. and Anderson, A.J., Catalase activities of Phanerochaete chrysosporium are not coordinately produced with ligninolytic metabolism: catalase from a white-rot fungus, Curr. Microbiol., 2001, vol. 42, pp. 8–11.
Lenartova, V., Holovska, K., and Javorsky, P., The influence of mercury on the antioxidant enzyme activity of rumen bacteria Streptococcus bovis and Selenomonas ruminantium, FEMS Microbiol Ecol., 1998, vol. 27, pp. 319–325.
Liu, J., Chen, H., Miller, D.S., Sauvedra, J.E., Keefer, L.K., Johnson, D.R., Klaassen, C.D., and Waalkes, M.P., Overexpression of glutathione s-transferase II and multi-drug resistance transport proteins is associated with acquired tolerance to inorganic arsenic, Mol. Pharmacol., 2001, vol. 60, pp. 302–309.
Lurie, S., Antioxidants, in Postharvest Oxidative Stress in Horticultural Crops, Hodges, D.M., Ed., New York: Food Products Press, 2003, pp. 131–150.
Mascher, R., Lippman, B., Holiinger, S., and Bergmann, H., Arsenate toxicity: effects on oxidative stress response molecules and enzymes in red clover plants, Plant Sci., 2002, vol. 63, pp. 961–969.
Montillet, J.L., Chamnongpol, S., Rustérucci, C., Dat, J., Van de Cotte, B., Agnel, J.-P., Battesti, C., Inzé, D., Van Breusegem, F., and Triantaphylidès, C., Fatty acid hydroperoxides and H2O2 in the execution of hypersensitive cell death in tobacco leaves, Plant Physiol., 2005, vol. 138, pp. 1516–1526.
Mylona, P.V., Polidoros, A.N., and Scandalios, J.G., Modulation of antioxidant response by arsenic in maize, Free Rad. Biol. Med., 1998, vol. 25, no. 4/5, pp. 576–585.
Nakano, Y. and Asada, K., Hydrogen peroxide is scavenged by ascorbate specific peroxide in spinach chloroplasts, Plant Cell Physiol., 1981, vol. 22, pp. 867–880.
Okamura, M., An improved method for determination of L-ascorbic acid and L-dehydroascorbic acid in blood plasma, Clin. Chem. Acta., 1980, vol. 103, pp. 259–268.
Ohkawa, H., Ohishi, N., and Yagi, K., Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction, Anal. Biochem., 1979, vol. 95, pp. 351–358.
Pnueli, L., Liang, H., Rozenberg, M., and Mittler, R. Growth suppression, altered stomatal responses and augmented induction of heat shock proteins in cytosolic ascorbate peroxidase (Apx1)-deficient Arabidopsis plants, Plant J., 2003, vol. 34, pp. 187–203.
Rai, A.K., Cyanobacterial Nitrogen Metabolism and Environmental Biotechnology, New Dehli: Narosa, 1997, pp. 73–87.
Rensing, C. and Rosen, B., Heavy metals cycle (arsenic, mercury, selenium, others), in Encyclopedia of Microbiology, Schaeter, M., Ed., Oxford: Elsevier, 2009, pp. 205–219.
Rippka, R., Derulles, J., Waterbury, J.B., Herdman, M., and Stanier, R.Y., Generic assignments, strain histories and properties of pure cultures of cyanobacteria, J. Gen. Microbiol., 1979, vol. 111, pp. 1–61.
Rosen, B.P., Biochemistry of arsenic detoxification, FEBS Lett., 2002, vol. 529, pp. 86–92.
Santos, C., Gaspar, C.A., Branco-Price, C., Teixeira, A., and Ferreira, R.B., Exposure of Lemna minor to arsenite: expression levels of components and intermediates of the ubiquitin/proteasome pathway, Plant Cell Physiol., 2006, vol. 47, pp. 1262–1273.
Schaedle, M. and Bassham, J.A., Chloroplast glutathione reductase, Plant Physiol., 1977, vol. 59, pp. 1011–1012.
Schmoger, M.E.V., Oven, M., and Grill, E., Detoxification of arsenic by phytochelatins in plants, Plant Physiol., 2000, vol. 122, pp. 793–802.
Singh, H.P., Batish, D.R., Kohlo, R.K., and Arora, K., Arsenic-induced root growth inhibition in mungbean (Phaseolus aureus Roxb.) is due to oxidative stress resulting from enhanced lipid peroxidation, Plant Growth Regul., 2007, vol. 53, pp. 65–73.
Smedley, P.L. and Kinniburgh, D.G., A review of the source, behaviour and distribution of arsenic in natural waters, Appl. Geochem., 2002, vol. 17, pp. 517–568.
Smirnoff, N., The role of active oxygen in the response of plants to water deficit and desiccation, New Phytol., 1993, vol. 125, pp. 27–58.
Srivastava, A.K., Bhargava, P., and Rai, L.C., Differential response of antioxidative defense system of Anabaena doliolum under arsenite and arsenate stress, J. Basic Microbiol., 2009, vol. 49, pp. S63–S72.
Srivastava, A.K., Bhargava, P., Mishra, Y., Shukla, B., and Rai, L.C., Effect of pretreatment of salt, copper and temperature on ultra-violet-B-induced antioxidants in diazotrophic cyanobacterium Anabaena doliolum, J. Basic Microbiol., 2006, vol. 46, pp. 135–144.
Stoeva, N., Berova, M., and Zlatev, Z., Effect of arsenic on some physiological parameters in bean plants, Biol. Plant., 2005, vol. 49, pp. 293–296.
Sundaram, S. and Soumya, K.K., Study of physiological and biochemical alterations in cyanobacterium under organic stress, Am. J. Plant. Physiol., 2011, vol. 6, pp. 1–16.
Thiel, T., Phosphate transport and arsenate resistance in the cyanobacterium Anabaena variabilis, J. Bacteriol., 1988, vol. 170, pp. 1143–1147.
Thordal-Christensen, H., Zhang, Z, Wei, Y., and Collinge, D.B., Subcellular localization of H2O2 in plants, H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction, The Plant J., 1997, vol. 11, pp. 1187–1192.
Turpeinen, R., Interactions between metals, microbes and plants: bioremediation of arsenic and lead contaminated soils, MSc Dissertation in Environmental Ecology, Fac. Sci., Univ. Helsinki, 2002.
Velikova, V., Yordanov, I., and Edreva, A., Oxidative stress and some antioxidant systems in acid rain-treated bean plants, protective role of exogenous polyamines, Plant Sci., 2000, vol.151, pp. 59–66.
Author information
Authors and Affiliations
Corresponding authors
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Dhuldhaj, U., Pandya, U. & Singh, S. Anti-Oxidative Response of Cyanobacterium Anabaena sp. strain PCC 7120 to Arsenite (As(III)). Microbiology 87, 848–856 (2018). https://doi.org/10.1134/S0026261718060097
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0026261718060097