Abstract
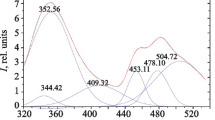
Ionic complexes of the composition [LnL2(NO3)2]2[Ln(NO3)5]3Me2CO (Ln = Sm (1), Eu (2), Tb (3), Dy (4)) with an optically active ligand L containing 1,10-phenanthroline and (+)−3-carene moieties are synthesized. According to the X-ray crystallographic data, the crystal structure of compound 2 is composed of complex [EuL2(NO3)2]+ cations (N6O4 polyhedron) and complex [Eu(NO3)5]2− anions (O10 polyhedron), and also Me2CO molecules. The L and NO3 ligands perform both tridentate and bidentate chelating functions respectively. Complexes 1–4 are isostructural and crystallize in the non-centrosymmetric space group P1; their magnetic properties are studied in the temperature range 2–300 K. The μeff values for 1–4 at 300 K are 3.14 μB, 6.08 μB, 16.76 μB, and 18.30 μB respectively and are typical of Ln3+ ions. For complex 3 significant anisotropy results in a nonlinear field dependence of the magnetization at 2 K. Complexes 1–4 exhibit metal-centered orange (Sm3+), red (Eu3+), green (Tb3+), and yellow (Dy3+) luminescence in the solid state at room temperature. Luminescence quantum yield decreases for solid samples in the order 2 > 1 > 3 ≈ 4.
Similar content being viewed by others
References
G. F. De Sa, O. L. Malta, C. de Mello Donega, A. M. Simas, R. L. Longo, P. A. Santa-Cruz, and E. F. da Silva Jr. Coord. Chem. Rev., 2000, 196, 165.
J.-C. G. Bünzli. Acc. Chem. Res., 2006, 39, 53.
J.-C. G. Bünzli and S. V. Eliseeva. Chem. Science, 2013, 4, 1939.
M. D. Allendorf, C. A. Bauer, R. K. Bhakta, and R. J. T. Houk. Chem. Soc. Rev., 2009, 38, 1330.
L. Armelao, S. Quici, F. Barigelletti, G. Accorsi, G. Bottaro, M. Cavazzini, and E. Tondello. Coord. Chem. Rev., 2010, 254, 487.
M. A. Katkova, A. G. Vitukhnovsky, and M. N. Bochkarev. Russ. Chem. Rev., 2005, 74, 1089.
M. A. Katkova and M. N. Bochkarev. Dalton Trans., 2010, 39, 6599.
M. N. Bochkarev, A. G. Vitukhnovsky, and M. A. Katkova. Organic Light Emitting Diodes (OLED). DEKOM: Nizhny Novgorod, 2011.
S. V. Larionov and Yu. A. Bryleva. Russ. J. Coord. Chem., 2016, 42, 293.
G. Accorsi, A. Listorti, K. Yoosaf, and N. Armaroli. Chem. Soc. Rev., 2009, 38, 1690.
D. L. Kepert, L. I. Semenova, A. N. Sobolev, and A. H. White. Aust. J. Chem., 1996, 49, 1005.
D.-Y. Wei, J.-L. Lin, and Y.-Q. Zheng. J. Coord. Chem., 2002, 55, 1259.
Y.-Q. Zheng, L.-X. Zhou, J.-L. Lin, and S.-W. Zhang. Z. Anorg. Allg. Chem., 2001, 627, 1643.
A. G. Mirochnik, B. V. Bukvetskii, P. A. Zhikhareva, and V. E. Karasev. Russ. J. Coord. Chem., 2001, 27, 443.
G. G. Sadikov, A. S. Antsyshkina, I. A. Kuznetsova, and M. N. Rodnikova. Crystallogr. Rep., 2006, 51, 47.
Z. Pan, G. Jia, C.-K. Duan, W.-Y. Wong, W.-T. Wong, and P. Tanner. Eur. J. Inorg. Chem., 2011, 5, 637.
G. G. Sadikov, A. S. Antsyshkina, M. N. Rodnikova, and I. A. Solonina. Crystallogr. Rep., 2009, 54, 48.
I. V. Ananyev, Yu. V. Nelyubina, L. N. Puntus, K. A. Lyssenko, and I. L. Eremenko, Russ. Chem. Bull., 2016, 65, 1178.
P. A. Tanner. Coord. Chem. Rev., 2010, 254, 3026.
L. N. Puntus and V. F. Zolin. Russ. J. Coord. Chem., 2003, 29, 574.
G. Muller. Dalton Trans., 2009, 44, 9692.
J. Crassous. Chem. Soc. Rev., 2009, 38, 830.
G. Muller, J.-C. G. Bunzli, J. P. Riehl, D. Suhr, A. von Zelewsky, and H. Mürner. Chem. Commun., 2002, 14, 1522.
J. Liu, X.-P. Zhang, T. Wu, B. B. Ma, T. W. Wang, C. H. Li, Y. Z. Li, and X. Z. You. Inorg. Chem., 2012, 51, 8649.
S. V. Larionov, Yu. A. Bryleva, L. A. Glinskaya, V. F. Plyusnin, A. S. Kupryakov, A. M. Agafontsev, A. V. Tkachev, A. S. Bogomyakov, D. A. Piryazev, and I. V. Korolkov. Dalton Trans., 2017, 46, 11440.
J. Lewis and T. D. O′Donoghue. Dalton Trans., 1980, 5, 736.
A. V. Tkachev and A. V. Rukavishnikov. Mendeleev Comm., 1992, 4, 161.
S. A. Popov, A. Yu. Denisov, Yu. V. Gatilov, I. Yu. Bagryanskaya, and A. V. Tkachev. Tetrahedron: Asymmetry, 1994, 3, 479.
G. M. Sheldrick. Acta Crystallogr., 2015, 71, 3.
F. W. Lewis, L. M. Harwood, M. J. Hudson, M. G. B. Drew, J. F. Desreux, G. Vidick, N. Bouslimani, G. Modolo, A. Wilden, M. Sypula, T.-H. Vu, and J.-P. Simonin. J. Am. Chem. Soc., 2011, 133, 13093.
M. Steppert, I. Císařová, T. Fanghänel, A. Geist, P. Lindqvist-Reis, P. Panak, P. Štěpnička, S. Trumm, and C. Walther. Inorg. Chem., 2012, 51, 591.
V. Tsaryuk, V. Zolin, L. Puntus, V. Savchenko, J. Legendziewicz, J. Sokolnicki, and R. Szostak. J. Alloys Compd., 2000, 300–301, 184.
I. R. Ferraro. J. Mol. Spectr., 1960, 4, 99.
K. Nakamoto. Infrared and Raman Spectra of Inorganic and Coordination Compounds Pt. A. USA, Wiley: Hoboken, 2009.
M. Andruh, E. Bakalbassis, O. Kahn, J. C. Trombe, and P. Porchers. Inorg. Chem., 1993, 32, 1616.
M. D. Regulacio, M. H. Publico, J. A. Vasquez, P. N. Myers, S. Gentry, M. Prushan, S.-W. Tam-Chang, and S. L. Stoll. Inorg. Chem., 2008, 47, 1512.
Acknowledgments
The authors are thankful to A.P. Zubareva and V.V. Ankudovich for the elemental analysis data, P.E. Plyusnin for the thermogravimetry data, N.I. Alferova for the IR spectra, and N.P. Korotkevich for the powder XRD data.
Funding
The study was supported by RFBR within scientific project No. 18-33-00239.
Author information
Authors and Affiliations
Corresponding author
Additional information
Conflict of Interests
The authors declare that they have no conflict of interests.
Russian Text © The Author(s), 2019, published in Zhurnal Strukturnoi Khimii, 2019, Vol. 60, No. 8, pp. 1366–1378.
Rights and permissions
About this article
Cite this article
Bryleva, Y.A., Glinskaya, L.A., Agafontsev, A.M. et al. Synthesis, Structure, Magnetic and Photoluminescent Properties of Lanthanide(III) Complexes with a Ligand Based on 1,10-Phenanthroline and (+)−3-Carene. J Struct Chem 60, 1314–1326 (2019). https://doi.org/10.1134/S0022476619080110
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476619080110