Abstract
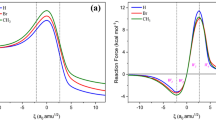
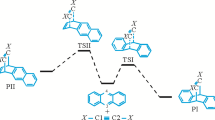
The mechanism and regioselectivity of the Diels–Alder cycloaddition reaction between 9- (methoxymethyl)anthracene and citraconic anhydride are explored using the valuable density functional theory (DFT) methods. The solvent effects are considered using the polarizable continuum model in the toluene solution. Due to a small electrophilicity difference of the reactants, the reaction has a low polar character. The investigated Diels–Alder reaction has a normal electron demand character. Depending on the respective position of substituents in the cycloadducts (head-to-head (ortho) or head-to-tail (meta)) the reaction can be progressed via two different pathways: ortho and meta. Due to a very high activation energy, the meta pathway is rejected. The product of the ortho pathway is demonstrated to be the final product of the reaction in the toluene solution. The obtained DFT results are in good agreement with the experimental results.
Similar content being viewed by others
References
D. L. Boger. Chem. Rev. (Washington, DC, US), 1986, 86, 781.
X. Jiang and R. Wang. Chem. Rev. (Washington, DC, US), 2013, 113, 5515.
K.–I. Takao, R. Munakata, and K.–I. Tadano. Chem. Rev. (Washington, DC, US), 2005, 105, 4779.
J. D. Winkler. Chem. Rev. (Washington, DC, US), 1996, 96, 167.
L. Song, G. Zhu, Y. Liu, B. Liu, and S. Qin. J. Am. Chem. Soc., 2015, 137, 13706.
E. V. Mironova, M. S. Dzyurkevich, O. A. Lodochnikova, D. B. Krivolapov, I. A. Litvinov, and V. V. Plemenkov. J. Struct. Chem., 2012, 53(2), 361–364.
M. Salakhov, O. Grechkina, and B. Bagmanov. J. Struct. Chem., 2010, 51(1), 16.
T. Barhoumi–Slimi, M. B. Dhia, M. Nsangou, M. El Gaied, and M. Khaddar. J. Struct. Chem., 2010, 51(2), 251.
D. L. Boger and S. M. Weinreb. Hetero Diels–Alder methodology in organic synthesis. Elsevier, 2012.
H. Oikawa and T. Tokiwano. Nat. Prod. Rep., 2004, 21, 321.
E. M. Stocking and R. M. Williams. Angew. Chem., Int. Ed., 2003, 42, 3078.
P. Buonora, J.–C. Olsen, and T. Oh. Tetrahedron, 2001, 57, 6099.
J. Barluenga, J. Joglar, F. González, and S. Fustero. Synlett, 1990, 1990, 129.
K. L. Burgess, N. J. Lajkiewicz, A. Sanyal, W. Yan, and J. K. Snyder. Org. Lett., 2005, 7, 31.
E. Ciganek. J. Org. Chem., 1980, 45, 1497.
S. Fukuzumi, T. Okamoto, and K. Ohkubo. J. Phys. Chem. A, 2003, 107, 5412.
B. Gacal, H. Durmaz, M. A. Tasdelen, G. Hizal, U. Tunca, Y. Yagci, and A. L. Demirel. Macromolecules, 2006, 39, 5330.
N. D. Khupse and A. Kumar. J. Phys. Chem. A, 2011, 115, 10211.
K. E. Kolb. J. Chem. Educ., 1989, 66, 955.
M. M. Kose, G. Yesilbag, and A. Sanyal. Org. Lett., 2008, 10, 2353.
K. E. Wise and R. A. Wheeler. J. Phys. Chem. A, 1999, 103, 8279.
A. Nierth, A. Y. Kobitski, G. U. Nienhaus, and A. Jäschke. J. Am. Chem. Soc., 2010, 132, 2646.
G. H. Schenk and D. R. Wirz. Anal. Chem., 1970, 42, 1754.
R. Khan, T. P. Singh, and M. D. Singh. Synlett, 2014, 25, 696.
L. R. Domingo, M. T. Picher, J. Andrés, and V. S. Safont. J. Org. Chem., 1997, 62, 1775.
S. Noorizadeh and H. Maihami. J. Mol. Struct.: THEOCHEM, 2006, 763, 133.
B. R. Beno, K. Houk, and D. A. Singleton. J. Am. Chem. Soc., 1996, 118, 9984.
L. R. Domingo, M. J. Aurell, P. Pérez, and R. Contreras. J. Phys. Chem. A, 2002, 106, 6871.
N. Çelebi–Ölçüm, A. Sanyal, and V. Aviyente. J. Org. Chem., 2009, 74, 2328.
S. S. Borisevich, A. V. Kovalskaya, I. P. Tsypysheva, and S. L. Khursan. J. Theor. Comput. Chem., 2014, 13, 1450048.
H. Chemouri and S. Mekelleche. J. Theor. Comput. Chem., 2006, 5, 197.
M. A. Fernández–Herrera, C. Zavala–Oseguera, J. L. Cabellos, J. Sandoval–Ramírez, L. R. Domingo, and G. Merino. J. Mol. Model., 2014, 20, 2207.
B. Szefczyk, T. Andruniów, and W. A. Sokalski. J. Mol. Model., 2008, 14, 727.
T. M. Barhoumi–Slimi, K. Essalah, M.a.K. Sanhoury, M. Ourévitch, and M. M. El Gaied. Struct. Chem., 2014, 25, 799.
C. Lee, W. Yang, and R. G. Parr. Phys. Rev. B, 1988, 37, 785.
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Rob, J. R. Cheeseman, J. A. Montgomery Jr., T. Vreven, K. N. Kudin, J. C. Burant, J. M. Millam, S. S. Iyengar, J. Tomasi, V. Barone, B. Mennucci, M. Cossi, G. Scalmani, N. Rega, G. A. Petersson, H. Nakatsuji, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, M. Klene, X. Li, J. E. Knox, H. P. Hrat–chian, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, P. Y. Ayala, K. Morokuma, G. A. Voth, P. Salvador, J. J. Dannenberg, V. G. Zakrzewski, S. Dapprich, A. D. Daniels, M. C. Strain, O. Farkas, D. K. Malick, A. D. Rabuck, K. Ra–ghavachari, J. B. Foresman, J. V. Ortiz, Q. Cui, A. G. Baboul, S. Clifford, J. Cioslowski, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. L. Martin, D. J. Fox, T. Keith, M. A. Al–Laham, C. Y. Peng, A. Nanayakkara, M. Challacombe, P. M. W. Gill, B. Johnson, W. Chen, M. W. Wong, C. Gonzalez, and J. A. Pople. Gaussian 03. Wallingford, CT: Gaussian, Inc., 2003.
C. Gonzalez and H. B. Schlegel. J. Phys. Chem., 1990, 94, 5523.
U. C. Singh and P. A. Kollman. J. Comput. Chem., 1984, 5, 129.
B. H. Besler, K. M. Merz, and P. A. Kollman. J. Comput. Chem., 1990, 11, 431.
F. De Proft, J. M. Martin, and P. Geerlings. Chem. Phys. Lett., 1996, 256, 400.
A. Bazian, S. A. Beyramabadi, A. Davoodnia, M. Pordel, and M. R. Bozorgmehr. Res. Chem. Intermed., 2016, 42, 6125.
W. Benchouk and S. Mekelleche. J. Mol. Struct.: THEOCHEM, 2008, 862, 1.
F. Moeinpour. Chin. J. Chem. Phys., 2010, 23, 165.
M. J. Aurell, L. R. Domingo, P. Pérez, and R. Contreras. Tetrahedron, 2004, 60, 11503.
P. Chattaraj. J. Phys. Chem. A, 2001, 105, 511.
R. G. Parr and W. Yang. Annu. Rev. Phys. Chem., 1995, 46, 701.
R. G. Parr and R. G. Pearson. J. Am. Chem. Soc., 1983, 105, 7512.
W. Yang and W. J. Mortier. J. Am. Chem. Soc., 1986, 108, 5708.
H. Chermette. J. Comput. Chem., 1999, 20, 129.
P. Geerlings, F. De Proft, and W. Langenaeker. Chem. Rev. (Washington, DC, US), 2003, 103, 1793.
R. G. Parr and W. Yang. J. Am. Chem. Soc., 1984, 106, 4049.
P. W. Ayers and R. G. Parr. J. Am. Chem. Soc., 2000, 122, 2010.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © 2018 A. Bazian, S. A. Beyramabadi, A. Davoodnia, M. R. Bozorgmehr, M. Pordel.
The text was submitted by the authors in English. Zhurnal Strukturnoi Khimii, Vol. 59, No. 8, pp. 1874–1880, November-December, 2018.
Rights and permissions
About this article
Cite this article
Bazian, A., Beyramabadi, S.A., Davoodnia, A. et al. A Theoretical Investigation on the Regioselectivity of the Diels–Alder Cycloaddition of 9-(Methoxymethyl) Anthracene And Citraconic Anhydride. J Struct Chem 59, 1810–1817 (2018). https://doi.org/10.1134/S0022476618080085
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476618080085