Abstract
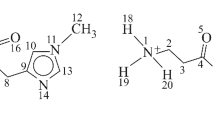
Complexes formed by leucine (Leu) and the dimer of sodium dodecyl sulfate (DDSNa)2 are studied by the quantum chemical method DFT using the hybrid exchange-correlation functional B97 with Grimme's dispersion correction D2 and the 6-311++G(2d,2p) basic set. The complexes formed by Leu and the dimer of DDSNa (considered as a micelle fragment) are studied in terms of their spatial structures and the energies of intermolecular interactions ЕIMI, depending on the penetration depth of Leu inside the micelle model. The highest energy ЕIMI is exhibited by the complex formed by the zwitterionic form of the amino acid and the hydrophilic part of sodium dodecyl sulfate. When the hydrophilic environment is replaced by the hydrophobic one, the intramolecular hydrogen bond in the amino acid changes in terms of its type and strength: N–H…O (weak) → N…H…O (strong) → N…O–H (weak). The energies of frontier orbitals and, therefore, redox properties of leucine also undergo changes in the course of the process.
Similar content being viewed by others
References
Antimicrobial Peptides: Methods and Protocols / Ed. by A. Giuliani and A. C. Rinaldi. USA, New York: Humana Press, 2010.
M. R. Bozorgmehr, M. Saberi, and H. Chegini. J. Mol. Liq., 2014, 199, 184.
I. Yu. Ponedel′kina, V. N. Odinokov, E. S. Vakhrusheva, M. T. Golikova, L. M. Khalilov, and U. M. Dzhemilev. Russ. J. Bioorg. Chem., 2005, 31, 82.
Y. Ding, Y. Shu, L. Ge, and R. Guo. Colloids Surf., A, 2007, 298, 163–169.
Z. Liu, X. Guo, Z. Feng, and L. Jia. J. Solution Chem., 2015, 44, 293.
H. D. Thaker, F. Sgolastra, D. Clements, R. W. Scott, and G. N. Tew. J. Med. Chem., 2011, 54, 2241.
I. M. Yermak and V. N. Davydova. Biochemistry (Moscow) supplement. Series A: Membrane and cell biology., 2008, 2(4), 279.
V. V. Andrushchenko, H. J. Vogel, and E. J. Prenner. Biochim. Biophys. Acta, 2007, 1768, 2447.
S. R. Dennison, J. Wallace, F. Harris, and D. A. Phoenix. Protein Pept. Lett., 2005, 12, 31.
A. Ali, N. A. Malik, and S. Uzair. Mol. Phys., 2014, 112, 2681.
M. S. Hossain, T. K. Biswas, and D. C. Kabiraz. J. Chem. Thermodyn., 2014, 71, 6.
B. Z. Idiyatullin, Y. F. Zuev, K. S. Potarikina, O. G. Us′yarov, and O. S. Zueva. Colloid J., 2013, 75, 532.
L. Zhu, Y. Han, M. Tian, and Y. Wang. Langmuir, 2013, 29, 12084.
S. V. Shilova, A. Y. Tret′yakova, and V. P. Barabanov. Russ. J. Phys. Chem. A, 2016, 90, 95.
Z. Yan, Q. Zhang, W.–W. Li, and J. Wang. J. Chem. Eng. Data, 2010, 55, 3560.
R. Saeed, M. Usman, A. Mansha, N. Rasool, S. A. R. Naqvi, A. F. Zahoor, H. M. A. Rahman, U. A. Rana, and E. Al–Zahrani. Colloids Surf. A, 2017, 512, 51.
V. G. Badelin, I. N. Mezhevoi, and E. Yu. Tyunina. Russ. J. Phys. Chem. A., 2017, 91(3), 523.
V. G. Badelin, E. Yu. Tyunina, and G. N. Tarasova. Zh. Fiz. Khim., 2017, 91(5), 862.
V. I. Smirnov and V. G. Badelin. J. Phys. Chem. A., 2017, 91, 1681.
S. V. Efimov, F. Kh. Karataeva, A. V. Aganov, S. Berger, and V. V. Klochkov. J. Mol. Struct., 2013, 1036, 298.
I. Z. Rakhmatullin, L. F. Galiullina, E. A. Klochkova, I. A. Latfullin, A. V. Aganov, and V. V. Klochkov. J. Mol. Struct., 2016, 1105, 25.
V. G. Zavodinskiy, A. A. Gnidenko, V. N. Davidova, and I. M. Ermak. Butlerov Comm., 2003, 4(2), 11.
D. E. Nolde, P. E. Volynskii, A. S. Arseniev, and R. G. Efremov. Russ. J. Bioorg. Chem., 2000, 26, 115.
A. S. Khamidullina, I. V. Vakulin, R. F. Talipov, and I. S. Shepelevich. J. Struct. Chem., 2005, 46(6), 985.
Z. Qiu, Y. Xia, H. Wang, and K. Diao. J. Struct. Chem., 2011, 52(3), 462.
N. I. Giricheva, M. S. Kurbatova, E. Yu. Tyunina, and V. G. Badelin. J. Struct. Chem., 2017, 58(8), 1604.
B. Tah, P. Pal, S. Roy, D. Dutta, S. Mishra, M. Ghosh, and G. B. Talapatra. Spectrochim. Acta, Part A, 2014, 129, 345.
J. P. Marcolongo and M. Mirenda. J. Chem. Educ., 2011, 88, 629.
B. Ch. Deka and P. Kr. Bhattacharyya. Comput. Theor. Chem., 2017, 1110, 40.
H.–D. Jakubke and H. Jeschkeit. Aminosäuren, Peptide, Proteine. Berlin, Akademie–Verlag, 1982.
S. Adhikari and T. Kar. J. Cryst. Growth., 2012, 356, 4.
V. Yu. Kurochkin, V. V. Chernikov, and T. D. Orlova. Russ. J. Phys. Chem. A., 2011, 85, 598.
A. K. Rai, X. Xu, Z. Lin, and D. K. Rai. Vib. Spectrosc., 2011, 56, 74.
N. A. Akhmedov, L. I. Ismailova, R. M. Abbasli, N. F. Akhmedov, and N. M. Godjaev. Russ. J. Bioorg. Chem., 2005, 31, 27.
S. G. Stepanian, A. Yu. Ivanov, and L. Adamowicz. Chem. Phys., 2013, 423, 20.
A. D. Becke. J. Chem. Phys., 1997, 107, 8554.
S. Grimme. J. Comp. Chem., 2006, 27, 1787.
R. Krishnan, J. S. Binkley, R. Seeger, and J. A. Pople. J. Chem. Phys., 1980, 72, 650.
M. J. Frisch, G. W. Truck, H. B. Schlegel, et al. Gaussian 09, Revision D.01. Gaussian, Inc., Wallingford CT, 2013.
F. Weinhold and C. R. Landis. Chem. Educ. Res. Pract. Eur., 2001, 2, 91.
N. V. Usol’tseva, А. I. Smirnova, N. V. Zharnikova, M. S. Kurbatova, N. I. Giricheva, and V. G. Badelin. Liq. Cryst. Their Appl., 2016, 16(2), 70.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © 2018 N. I. Giricheva, M. S. Kurbatova, E. Yu. Tyunina, V. P. Barannikov.
Translated from Zhurnal Strukturnoi Khimii, Vol. 59, No. 8, pp. 1834–1841, November-December, 2018.
Rights and permissions
About this article
Cite this article
Giricheva, N.I., Kurbatova, M.S., Tyunina, E.Y. et al. A Quantum Chemical Simulation of the Interaction Between Leucine and the Dimer of Sodium Dodecyl Sulphate. J Struct Chem 59, 1768–1775 (2018). https://doi.org/10.1134/S0022476618080024
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476618080024