Abstract
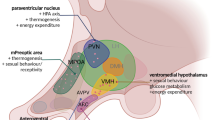
Most of regulatory effects of leptin on feeding behavior and energy metabolism are implemented via the hypothalamic leptin system. Attenuation of its activity leads to hyperphagia, obesity and other metabolic disorders. To prevent these pathological conditions, it is necessary to develop approaches aimed at restoring the leptin system. Most promising of them is creating efficient inhibitors of protein phosphotyrosine phosphatase PTP1B, a negative regulator of leptin signaling. The aim of this work was to synthesize a new compound, ethyl-3-(hydroxymethyl)-4-oxo-1,4-dihydrocinnoline-6-carboxylate (PI-04), a 4-oxo-1,4-dihydrocinnoline derivative with a PTP1B inhibitor activity, and to study its effect on IRS2- and STAT3-dependent leptin pathways in culture of hypothalamic neurons isolated from 18-day-old rat embryos. It was shown that PI-04 enhances the stimulatory effect of leptin on phosphorylation of the IRS2 protein at the Ser731 residue and of the STAT3 transcriptional factor at the Tyr705 residue, suggesting a potentiation of functional responses of hypothalamic neurons to leptin in the presence of this compound. The potentiating effect of PI-04 on leptin signaling was implemented at micromolar concentrations when this substance had virtually no effect on neuronal survival. The data obtained are promising in terms of creating phosphatase PTP1B inhibitors based on 4-oxo-1,4-dihydrocinnoline, as well as the possibility of their further use to prevent and correct metabolic disorders caused by attenuated activity of the hypothalamic leptin system.
Similar content being viewed by others
Abbreviations
- LepR:
-
leptin receptor
- MTT:
-
3-(4,5-dimethylthiazol)-2,5-diphenyl-2-tetrazolium bromide
- AKT:
-
serine/threonine protein kinase B (AKT-kinase)
- PTP1B:
-
protein phosphotyrosine phosphatase 1B
- STAT3 and STAT5:
-
signal transducers and type 3 and 5 transcriptional activators
References
Friedman, J.M. and Halaas, J.L., Leptin and the regulation of body weight in mammals, Nature, 1998, vol. 395, pp. 763–770.
Zhou, Y. and Rui, L., Leptin signaling and leptin resistance, Front. Med., 2013, vol. 7, pp. 207–222.
Shpakov, A.O., Derkach, K.V., and Berstein, L.M., Brain signaling systems in the type 2 diabetes and metabolic syndrome: promising target to treat and prevent these diseases, Future Science OA(FSO), 2015, vol. 1, FSO25. doi: 10.4155/fso.15.23
Morris, D.L. and Rui, L., Recent advances in understanding leptin signaling and leptin resistance, Am. J. Physiol., 2009, vol. 297, pp. 1247–1259.
Myers, M.G., Leibel, R.L., Seeley, R.J., and Schwartz, M.W., Obesity and leptin resistance: distinguishing cause from effect, Trends Endocrinol. Metab., 2010, vol. 21, pp. 643–651.
El-Haschimi, K., Pierroz, D.D., Hileman, S.M., Bjorbaek, C., and Flier, J.S., Two defects contribute to hypothalamic leptin resistance in mice with diet-induced obesity, J. Clin. Invest., 2000, vol. 105, pp. 1827–1832.
Banks, W.A. and Farrell, C.L., Impaired transport of leptin across the blood-brain barrier in obesity is acquired and reversible, Am. J. Physiol., 2003, vol. 285, pp. 10–15.
Belouzard, S., Delcroix, D., and Rouille, Y., Low levels of expression of leptin receptor at the cell surface result from constitutive endocytosis and intracellular retention in the biosynthetic pathway, J. Biol. Chem., 2004, vol. 279, pp. 28 499–28 508.
Hosoi, T., Sasaki, M., Miyahara, T., Hashimoto, C., Matsuo, S., Yoshii, M., and Ozawa, K., Endoplasmic reticulum stress induces leptin resistance, Mol. Pharmacol., 2008, vol. 74, pp. 1610–1619.
Coppari, R. and Bjorbaek, C., Leptin revisited: its mechanism of action and potential for treating diabetes, Nat. Rev. Drug Discov., 2012, vol. 11, pp. 692–708.
Cheng, A., Uetani, N., Simoncic, P.D., Chaubey, V.P., Lee-Loy, A., McGlade, C.J., Kennedy, B.P., and Tremblay, M.L., Attenuation of leptin action and regulation of obesity by protein tyrosine phosphatase 1B, Dev. Cell, 2002, vol. 2, pp. 497–503.
Bence, K.K., Delibegovic, M., Xue, B., Gorgun, C.Z., Hotamisligil, G.S., Neel, B.G., and Kahn, B.B., Neuronal PTP1B regulates body weight, adiposity and leptin action, Nat. Med., 2006, vol. 12, pp. 917–924.
Loh, K., Fukushima, A., Zhang, X., Galic, S., Briggs, D., Enriori, P.J., Simonds, S., Wiede, F., Reichenbach, A., Hauser, C., Sims, N.A., Bence, K.K., Zhang, S., Zhang, Z.Y., Kahn, B.B., Neel, B.G., Andrews, Z.B., Cowley, M.A., and Tiganis, T., Elevated hypothalamic TCPTP in obesity contributes to cellular leptin resistance, Cell. Metab., 2011, vol. 14, pp. 684–699.
Tsou, R.C. and Bence, K.K., Central regulation of metabolism by protein tyrosine phosphatases, Front. Neurosci., 2013, vol. 6, p. 192.
Kaszubska, W., Falls, H.D., Schaefer, V.G., Haasch, D., Frost, L., Hessler, P., Kroeger, P.E., White, D.W., Jirousek, M.R., and Trevillyan, J.M., Protein tyrosine phosphatase 1B negatively regulates leptin signaling in a hypothalamic cell line, Mol. Cell. Endocrinol., 2002, vol. 195, pp. 109–118.
Tsou, R.C., Zimmer, D.J., De Jonghe, B.C., and Bence, K.K., Deficiency of PTP1B in leptin receptor-expressing neurons leads to decreased body weight and adiposity in mice, Endocrinology, 2012, vol. 153, pp. 4227–4237.
Cho, H., Protein tyrosine phosphatase 1B (PTP1B) and obesity, Vitam. Horm., 2013, vol. 91, pp. 405–424.
Zhang, S. and Zhang, Z.Y., PTP1B as a drug target: recent developments in PTP1B inhibitor discovery, Drug Discov. Today, 2007, vol. 12, pp. 373–381.
Sorokoumov, V.N. and Shpakov, A.O., Protein phosphotyrosine phosphatase 1B: structure, functions, role in the development of metabolic disorders, and their correction by the enzyme inhibitors, J. Evol. Biochem. Physiol., 2017, vol. 53, no. 4, pp. 259–270.
Zhi, Y., Gao, L.X., Jin, Y., Tang, C.L., Li, J.Y., Li, J., and Long, Y.Q., 4-Quinolone-3-carboxylic acids as cell-permeable inhibitors of protein tyrosine phosphatase 1B, Bioorg. Med. Chem., 2014, vol. 22, pp. 3670–3683.
Bakke, J. and Haj, F.G., Protein-tyrosine phosphatase 1B substrates and metabolic regulation, Semin. Cell Dev. Biol., 2015, vol. 37, pp. 58–65.
Shpakov, A.O., The brain leptin signaling system and its functional state in metabolic syndrome and type 2 diabetes mellitus, J. Evol. Biochem. Physiol., 2016, vol. 52, no. 3, pp. 177–195.
Bhattarai, B.R., Kafle, B., Hwang, J.-S., Ham, S.W., Lee, K.-H., Park, H., Han, I.O., and Cho, H., Novel thiazolidine dione derivatives with anti-obesity effects: dual action as PTP1B inhibitors and PPAR-? activators, Bioorg. Med. Chem. Lett., 2010, vol. 20, pp. 6758–6763.
Ito, M., Fukuda, S., Sakata, S., Morinaga, H., and Ohta, T., Pharmacological effects of JTT-551, a novel protein tyrosine phosphatase 1B inhibitor, in diet-induced obesity mice, J. Diabetes Res., 2014, vol. 2014. doi: 10.1155/2014/680348
Niswender, K.D., Morton, G.J., Stearns, W.H., Rhodes, C.J., Myers, M.G., Jr., and Schwartz, M.W., Intracellular signalling. Key enzyme in leptin-induced anorexia, Nature, 2001, vol. 413, pp. 794–795.
Lin, X., Taguchi, A., Park, S., Kushner, J.A., Li, F., Li, Y., and White, M.F., Dysregulation of insulin receptor substrate 2 in beta cells and brain causes obesity and diabetes, J. Clin. Invest., 2004, vol. 114, pp. 908–916.
Jiang, L., You, J., Yu, X., Gonzalez, L., Yu, Y., Wang, Q., Yang, G., Li, W., Li, C., and Liu, Y., Tyrosine-dependent and-independent actions of leptin receptor in control of energy balance and glucose homeostasis, Proc. Natl. Acad. Sci. USA, 2008, vol. 105, pp. 18 619–18 624.
Piper, M.L., Unger, E.K., Myers, M.G., Jr., and Xu, A.W., Specific physiological roles for signal transducer and activator of transcription 3 in leptin receptor-expressing neurons, Mol. Endocrinol., 2008, vol. 22, pp. 751–759.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © I.O. Zakharova, V.N. Sorokoumov, L.V. Bayunova, K.V. Derkach, A.O. Shpakov, 2018, published in Zhurnal Evolyutsionnoi Biokhimii i Fiziologii, 2018, Vol. 54, No. 4, pp. 240–246.
Rights and permissions
About this article
Cite this article
Zakharova, I.O., Sorokoumov, V.N., Bayunova, L.V. et al. 4-oxo-1,4-dihydrocinnoline Derivative with Phosphatase 1B Inhibitor Activity Enhances Leptin Signal Transduction in Hypothalamic Neurons. J Evol Biochem Phys 54, 273–280 (2018). https://doi.org/10.1134/S0022093018040038
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022093018040038