Abstract
Human papillomaviruses of high carcinogenic risk (HR HPVs) are major etiological agents of malignant diseases of the cervix, vulva, penis, anal canal, larynx, head, and neck. Prophylactic vaccination against HPV, which mainly covers girls and women under 25, does not prevent vertical and horizontal HPV transmission in infants and children and does not have a therapeutic effect. As a result, a significant proportion of the population is not protected from the HPV infection and development of HPV-associated neoplastic transformation and cancer, which indicates the need for development and intro- duction of therapeutic HPV vaccines. Unlike prophylactic vaccines aimed at the formation of virus-neutralizing antibodies, therapeutic vaccines elicit cellular immune response leading to the elimination of infected and malignant cells expressing viral proteins. The ideal targets for vaccine immunotherapy are highly conserved HR HPV oncoproteins E6 and E7 expressed in precancerous and tumor tissues. Here, we describe expression of these proteins during different stages of HPV infection, their antigenic and immunogenic properties, and T-cell epitopes, the response to which correlates with natural regression of HPV-induced neoplastic changes. The review describes patterns of E6 and E7 oncoproteins presentation to the immune system as components of candidate vaccines along with the results of the most promising preclinical trials and animal models used in these trials. Special attention is paid to vaccine candidates which have shown efficacy in clinical trials in patients with HPV-associated neoplastic changes.
Article PDF
Similar content being viewed by others
Abbreviations
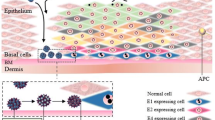
- APC:
-
antigen-presenting cell
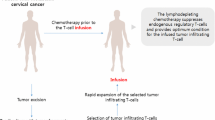
- CC:
-
cervical cancer
- CTL:
-
cytotoxic T-lymphocyte
- DC:
-
dendritic cell
- HLA:
-
human leukocyte antigen
- HR HPV:
-
human papillomavirus of high carcinogenic risk
- HSIL:
-
high grade squamous intraepithelial lesion
- ICI:
-
immune checkpoint inhibitor
- LLO:
-
listeriolysin O
- LSIL:
-
low-grade squamous intraepithelial lesion
- MHC:
-
main histocompatibility complex
- MVA:
-
modified vaccinia virus Ankara
- ORF:
-
open reading frame
- SCCHN:
-
squamous cell carcinoma of the head and neck
- SFV:
-
Semliki Forest virus
- SLPs:
-
long (including overlapping) synthetic peptides
- TAA:
-
tumor associated antigen
- TCR:
-
T-cell receptor
- TLR:
-
toll-like receptor
References
Arbyn, M., Xu, L., Simoens, C., and Martin-Hirsch, P. P. L. (2018) Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors, Cochrane Database Syst. Rev., 5, CD009069, doi: 10.1002/14651858.CD009069.pub3.
Prilepskaya, V. N., Zardiashvili, M. D., Khlebkova, Yu. S., and Nekrasova, M. E. (2016) Vaccination against HPV-associated diseases and cervical cancer: theoretical and practical aspects, Med. Sovet, 12, 120–125, doi: 10.21518/2079.701X-2016-12-120-125.
Cutts, F. T., Franceschi, S., Goldie, S., Castellsague, X., de Sanjose, S., Garnett, G., Edmunds, W. J., Claeys, P., Goldenthal, K. L., Harper, D. M., and Markowitz, L. (2007) Human papillomavirus and HPV vaccines: a review, Bull. World Health Organ., 85, 719–726, doi: 10.2471/BLT.06.038414.
Vonsky, M. S., Shabaeva, M. G., Runov, A. L., Lebedeva, N. N., Palefsky, D., and Isaguliants, M. G. (2019) Carcinogenesis associated with human papillomavirus infection. Mechanisms and potential for immunotherapy, Biochemistry (Moscow), 84, 782–799.
Yang, A., Farmer, E., Wu, T. C., and Hung, C. F. (2016) Perspectives for therapeutic HPV vaccine development, J. Biomed. Sci., 23, 75, doi: 10.1186/s12929-016-0293-9.
Chabeda, A., Yanez, R., Jr., Lamprecht, R., Meyers, A. E., Rybicki, E. P., and Hitzeroth, I. I. (2017) Therapeutic vaccines for high-risk HPV-associated diseases, Papillomavirus Res., 5, 46–58, doi: 10.1016/j.pvr.2017.12.006.
Petrova, G. V., Gretsova, O. P., Shahzadova, A. O., Prostov, M. Yu., Prostov, Yu. I., and Samsonov, Yu. V. (2018) in Malignant Tumors in Russia in 2017. Morbidity and Mortality (Kaprin, A. D., Starinsky, V. V., and Petrova, G. V., eds.) Hertsen Moscow Oncology Research Center, Moscow, pp. 4–130.
Alyautdina, O. S., and Darmostukova, M. A. (2018) Modern aspects of human papillomavirus vaccination, Bezopas. Risk Farmakoter., 6, 111–117, doi: 10.30895/2312.7821-2018-6-3-111-117.
Trimble, C. L., Morrow, M. P., Kraynyak, K. A., Shen, X., Dallas, M., Yan, J., Edwards, L., Parker, R. L., Denny, L., Giffear, M., Brown, A. S., Marcozzi-Pierce, K., Shah, D., Slager, A. M., Sylvester, A. J., Khan, A., Broderick, K. E., Juba, R. J., Herring, T. A., Boyer, J., Lee, J., Sardesai, N. Y., Weiner, D. B., and Bagarazzi, M. L. (2015) Safety, efficacy, and immunogenicity of VGX-3100, a therapeutic synthetic DNA vaccine targeting human papillomavirus 16 and 18.E6 and E7 proteins for cervical intraepithelial neoplasia 2.3: a randomised, double-blind, placebo-controlled phase 2. trial, Lancet, 386, 2078–2088, doi: 10.1016/S01406736(15)00239-1.
Wong, K. K., Li, W. A., Mooney, D. J., and Dranoff, G. (2016) Advances in therapeutic cancer vaccines, Adv. Immunol., 130, 191–249, doi: 10.1016/bs.ai.2015.12.001.
Kash, N., Lee, M. A., Kollipara, R., Downing, C., Guidry, J., and Tyring, S. K. (2015) Safety and efficacy data on vaccines and immunization to human papillomavirus, J. Clin. Med., 4, 614–633, doi: 10.3390/jcm4040614.
Ma, B., Maraj, B., Tran, N. P., Knoff, J., Chen, A., Alvarez, R. D., Hung, C. F., and Wu, T. C. (2012) Emerging human papillomavirus vaccines, Expert Opin. Emerg. Drugs, 17, 469–492, doi: 10.1517/14728214.2012.744393.
Wise-Draper, T. M., and Wells, S. I. (2008) Papillomavirus E6 and E7 proteins and their cellular targets, Front. Biosci., 13, 1003–1017.
Vande Pol, S. B., and Klingelhutz, A. J. (2013) Papillomavirus E6 oncoproteins, Virology, 445, 115–137, doi: 10.1016/j.virol.2013.04.026.
Miller, J., Dakic, A., Chen, R., Palechor-Ceron, N., Dai, Y., Kallakury, B., Schlegel, R., and Liu, X. (2013) HPV16 E7 protein and hTERT proteins defective for telomere maintenance cooperate to immortalize human keratinocytes, PLoS Pathog., 9, e1003284, doi: 10.1371/journal.ppat.1003284.
Edmonds, C., and Vousden, K. H. (1989) A point mutational analysis of human papillomavirus type 16 E7 protein, J. Virol., 63, 2650–2656.
Zine El Abidine, A., Tomaic, V., Bel Haj Rhouma, R., Massimi, P., Guizani, I., Boubaker, S., Ennaifer, E., and Banks, L. (2017) A naturally occurring variant of HPV-16 E7 exerts increased transforming activity through acquisition of an additional phospho-acceptor site, Virology, 500, 218–225, doi: 10.1016/j.virol.2016.10.023.
Doorbar, J. (2016) Model systems of human papillomavirus-associated disease, J. Pathol., 238, 166–179, doi: 10.1002/path.4656.
Song, S., Pitot, H. C., and Lambert, P. F. (1999) The human papillomavirus type 16 E6 gene alone is sufficient to induce carcinomas in transgenic animals, J. Virol., 73, 5887–5893.
De Azambuja, K., Barman, P., Toyama, J., David, E. D., Lawson, G. W., Williams, L. K., Chua, K., Lee, D., Kehoe, J. J., Brodkorb, A., Schwiebert, R., Kitchen, S., Bhimani, A., and Wiley, D. J. (2014) Validation of an HPV16-mediated carcinogenesis mouse model, In vivo, 28, 761–767.
Iuliano, M., Mangino, G., Chiantore, M. V., Zangrillo, M. S., Accardi, R., Tommasino, M., Fiorucci, G., and Romeo, G. (2018) Human papillomavirus E6 and E7 oncoproteins affect the cell microenvironment by classical secretion and extracellular vesicles delivery of inflammatory mediators, Cytokine, 106, 182–189, doi: 10.1016/j.cyto.2017.11.003.
Mirabello, L., Yeager, M., Yu, K., Clifford, G. M., Xiao, Y., Zhu, B., Cullen, M., Boland, J. F., Wentzensen, N., Nelson, C. W., Raine- Bennett, T., Chen, Z., Bass, S., Song, L., Yang, Q., Steinberg, M., Burdett, L., Dean, M., Roberson, D., Mitchell, J., Lorey, T., Franceschi, S., Castle, P. E., Walker, J., Zuna, R., Kreimer, A. R., Beachler, D. C., Hildesheim, A., Gonzalez, P., Porras, C., Burk, R. D., and Schiffman, M. (2017) HPV16 E7 genetic conservation is critical to carcinogenesis, Cell, 170, 1164–1174, doi: 10.1016/j.cell.2017.08.001.
Zhang, G. L., Riemer, A. B., Keskin, D. B., Chitkushev, L., Reinherz, E. L., and Brusic, V. (2014) HPVdb: a data mining system for knowledge discovery in human papillomavirus with applications in T-cell immunology and vaccinology, Database (Oxford), 2014, bau031, doi: 10.1093/ database/bau031.
Chan, P. K., Liu, S. J., Cheung, J. L., Cheung, T. H., Yeo, W., Chong, P., and Man, S. (2011) T-cell response to human papillomavirus type 52 L1, E6, and E7 peptides in women with transient infection, cervical intraepithelial neoplasia, and invasive cancer, J. Med. Virol., 83, 1023–1030, doi: 10.1002/jmv.21889.
Nakagawa, M., Kim, K. H., Gillam, T. M., and Moscicki, A. B. (2006) HLA class I binding promiscuity of the CD8 T-cell epitopes of human papillomavirus type 16 E6 protein, J. Virol., 81, 1412–1423, doi: 10.1128/JVI.01768-06.
De Vos van Steenwijk, P. J., Heusinkveld, M., Ramwadhdoebe, T. H., Lowik, M. J., van der Hulst, J. M., Goedemans, R., Piersma, S. J., Kenter, G. G., and van der Burg, S. H. (2010) An unexpectedly large polyclonal repertoire of HPV-specific T-cells is poised for action in patients with cervical cancer, Cancer Res., 70, 2707–2717, doi: 10.1158/0008-5472.CAN-09-4299.
Grabowska, A. K., Kaufmann, A. M., and Riemer, A. B. (2015) Identification of promiscuous HPV16-derived T helper cell epitopes for therapeutic HPV vaccine design, Int. J. Cancer, 136, 212–224, doi: 10.1002/ijc.28968.
Evans, M., Borysiewicz, L. K., Evans, A. S., Rowe, M., Jones, M., Gileadi, U., Cerundolo, V., and Man, S. (2001) Antigen processing defects in cervical carcinomas limit the presentation of a CTL epitope from human papillomavirus 16 E6, J. Immunol., 167, 5420–5428, doi: 10.4049/jimmunol.167.9.5420.
Peng, S., Trimble, C., Wu, L., Pardoll, D., Roden, R., Hung, C. F., and Wu, T. C. (2007) HLA-DQB1*02-restricted HPV-16 E7 peptide-specific CD4+ T-cell immune responses correlate with regression of HPV-16-associated high-grade squamous intraepithelial lesions, Clin. Cancer Res., 13, 2479–2487, doi: 10.1158/1078-0432.CCR-06-2916.
Van den Hende, M., Redeker, A., Kwappenberg, K. M., Franken, K. L., Drijfhout, J. W., Oostendorp, J., Valentijn, A. R., Fathers, L. M., Welters, M. J., Melief, C. J., Kenter, G. G., van der Burg, S. H., and Offringa, R. (2010) Evaluation of immunological cross-reactivity between clade A9 high-risk human papillomavirus types on the basis of E6-specific CD4+ memory T-cell responses, J. Infect. Dis., 202, 1200–1211, doi: 10.1086/656367.
Kim, K. H., Dishongh, R., Santin, A. D., Cannon, M. J., Bellone, S., and Nakagawa, M. (2006) Recognition of a cervical cancer derived tumor cell line by a human papillomavirus type 16 E6 52-61-specific CD8 T-cell clone, Cancer Immun., 6, 9.
Christensen, N. D., Budgeon, L. R., Cladel, N. M., and Hu, J. (2016) Recent advances in preclinical model systems for papillomaviruses, Virus Res., 231, 108–118, doi: 10.1016/j.virusres.2016.12.004.
Lin, K. Y., Guarnieri, F. G., Staveley-O’ Carroll, K. F., Levitsky, H. I., August, J. T., Pardoll, D. M., and Wu, T. C. (1996) Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen, Cancer Res., 56, 21–26.
Cheng, W. F., Hung, C. F., Lin, K. Y., Ling, M., Juang, J., He, L., Lin, C. T., and Wu, T. C. (2003) CD8+ T-cells, NK cells and IFN-gamma are important for control of tumor with downregulated MHC class I expression by DNA vaccination, Gene Ther., 10, 1311–1320, doi: 10.1038/sj.gt.330198.
Beyranvand, N. E., van der Sluis, T. C., van Duikeren, S., Yagita, H., Janssen, G. M., van Veelen, P. A., Melief, C. J., van der Burg, S. H., and Arens, R. (2016) Tumor eradication by cisplatin is sustained by CD80/86-mediated costimulation of CD8+ T-cells, Cancer Res., 76, 6017–6029, doi: 10.1158/0008-5472.CAN-16-0881.
Liu, Z., Zhou, H., Wang, W., Fu, Y. X., and Zhu, M. (2016) A novel dendritic cell targeting HPV16 E7 synthetic vaccine in combination with PD-L1 blockade elicits therapeutic antitumor immunity in mice, Oncoimmunology, 5, e1147641, doi: 10.1080/2162402X.2016.1147641.
Mkrtichyan, M., Chong, N., Abu, E. R., Wallecha, A., Singh, R., Rothman, J., and Khleif, S. N. (2013) Anti-PD-1 antibody significantly increases therapeutic efficacy of Listeria monocytogenes (Lm)-LLO immunotherapy, J. Immunother. Cancer, 1, 15, doi: 10.1186/2051-1426-1-15.
Song, L., Yang, M. C., Knoff, J., Wu, T. C., and Hung, C. F. (2014) Cancer immunotherapy employing an innovative strategy to enhance CD4+ T-cell help in the tumor microenvironment, PloS One, 9, e115711, doi: 10.1371/journal.pone.0115711.
Peng, S., Qiu, J., Yang, A., Yang, B., Jeang, J., Wang, J. W., Chang, Y. N., Brayton, C., Roden, R. B., Hung, C. F., and Wu, T. C. (2016) Optimization of heterologous DNA-prime, protein boost regimens and site of vaccination to enhance therapeutic immunity against human papillo-mavirus-associated disease, Cell Biosci., 6, 16, doi: 10.1186/s13578-016-0080-z.
Nakagawa, M., Stites, D. P., Patel, S., Farhat, S., Scott, M., Hills, N. K., Palefsky, J. M., and Moscicki, A. B. (2000) Persistence of human papillomavirus type 16 infection is associated with lack of cytotoxic T lymphocyte response to the E6 antigens, J. Infect. Dis., 182, 595–598, doi: 10.1086/315706.
Baldwin, P. J., van der Burg, S. H., Boswell, C. M., Offringa, R., Hickling, J. K., Dobson, J., Roberts, J. S., Latimer, J. A., Moseley, R. P., Coleman, N., Stanley, M. A., and Sterling, J. C. (2003) Vaccinia-expressed human papillomavirus 16 and 18 e6 and e7 as a therapeutic vaccination for vulval and vaginal intraepithelial neoplasia, Clin. Cancer Res., 9, 5205–5213.
Cordeiro, M. N., De Lima, R. C. P., Paolini, F., Melo, A. R. D. S., Campos, A. P. F., Venuti, A., and De Freitas, A. C. (2018) Current research into novel therapeutic vaccines against cervical cancer, Expert Rev. Anticancer Ther., 18, 365–376, doi: 10.1080/14737140.2018.1445527.
Kawana, K., Adachi, K., Kojima, S., Taguchi, A., Tomio, K., Yamashita, A., Nishida, H., Nagasaka, K., Arimoto, T., Yokoyama, T., Wada- Hiraike, O., Oda, K., Sewaki, T., Osuga, Y., and Fujii, T. (2014) Oral vaccination against HPV E7 for treatment of cervical intraepithelial neoplasia grade 3 (CIN3) elicits E7-specific mucosal immunity in the cervix of CIN3 patients, Vaccine, 32, 6233–6239, doi: 10.1016/j.vaccine.2014.09.020.
Komatsu, A., Igimi, S., and Kawana, K. (2018) Optimization of human papillomavirus (HPV) type 16 E7-expressing lactobacillus-based vaccine for induction of mucosal E7-specific IFNγ-producing cells, Vaccine, 36, 3423–3426, doi: 10.1016/j.vaccine.2018.05.009.
Peters, C., and Paterson, Y. (2003) Enhancing the immunogenicity of bioengineered Listeria monocytogenes by passaging through live animal hosts, Vaccine, 21, 1187–1194, doi: 10.1016/S0264-410X(02)00554-6.
Chen, Z., Ozbun, L., Chong, N., Wallecha, A., Berzofsky, J. A., and Khleif, S. N. (2014) Episomal expression of truncated listeriolysin O in LmddA-LLO-E7 vaccine enhances antitumor efficacy by preferentially inducing expansions of CD4+FoxP3− and CD8+ T-cells, Cancer Immunol. Res., 2, 911–922, doi: 10.1158/2326-6066.CIR-13-0197.
Maciag, P. C., Radulovic, S., and Rothman, J. (2009) The first clinical use of a live-attenuated Listeria monocytogenes vaccine: a phase I safety study of Lm-LLO-E7 in patients with advanced carcinoma of the cervix, Vaccine, 27, 3975–3983, doi: 10.1016/j.vaccine.2009.04.041.
Miles, B. A., Monk, B. J., and Safran, H. P. (2017) Mechanistic insights into ADXS11-001 human papillo-mavirus-associated cancer immunotherapy, Gynecol. Oncol. Res. Pract., 4, 9, doi: 10.1186/s40661-017-0046-9.
Kaufmann, A. M., Stern, P. L., Rankin, E. M., Sommer, H., Nuessler, V., Schneider, A., Adams, M., Onon, T. S., Bauknecht, T., Wagner, U., Kroon, K., Hickling, J., Boswell, C. M., Stacey, S. N., Kitchener, H. C., Gillard, J., Wanders, J., Roberts, J. S., and Zwierzina, H. (2002) Safety and immunogenicity of TA-HPV, a recombinant vaccinia virus expressing modified human papillomavirus (HPV)-16 and HPV-18 E6 and E7 genes, in women with progressive cervical cancer, Clin. Cancer Res., 8, 3676–3685.
Brun, J. L., Dalstein, V., Leveque, J., Mathevet, P., Raulic, P., Baldauf, J. J., Scholl, S., Huynh, B., Douvier, S., Riethmuller, D., Clavel, C., Birembaut, P., Calenda, V., Baudin, M., and Bory, J. P. (2011) Regression of high-grade cervical intraepithelial neoplasia with TG4001 targeted immunotherapy, Am. J. Obst. Gynecol., 204, e1–e8, doi: 10.1016/j.ajog.2010.09.020.
Rosales, R., Lopez-Contreras, M., Rosales, C., Magallanes-Molina, J. R., Gonzalez-Vergara, R., Arroyo-Cazarez, J. M., Ricardez-Arenas, A., Del Follo-Valencia, A., Padilla-Arriaga, S., Guerrero, M. V., Pirez, M. A., Arellano-Fiore, C., and Villarreal, F. (2014) Regression of human papillomavirus intraepithelial lesions is induced by MVA E2 therapeutic vaccine, Hum. Gene Ther., 25, 1035–1049, doi: 10.1089/hum.2014.024.
Vujadinovic, M., and Vellinga, J. (2018) Progress in adenoviral capsid-display vaccines, Biomedicines, 6, E81, doi: 10.3390/biomedicines6030081.
Gomez-Gutierrez, J. G., Elpek, K. G., Montes de Oca-Luna, R., Shirwan, H., Sam Zhou, H., and McMasters, K. M. (2007) Vaccination with an adenoviral vector expressing calreticulin-human papillomavirus 16 E7 fusion protein eradicates E7 expressing established tumors in mice, Cancer Immunol. Immunother., 56, 997–1007, doi: 10.1007/ s00262-006-0247-2.
Daemen, T., Riezebos-Brilman, A., Regts, J., Dontje, B., van der Zee, A., and Wilschut, J. (2004) Superior therapeutic efficacy of alphavirus-mediated immunization against human papilloma virus type 16 antigens in a murine tumour model: effects of the route of immunization, Antivir. Ther., 9, 733–742.
Van de Wall, S., Walczak, M., van Rooij, N., Hoogeboom, B. N., Meijerhof, T., Nijman, H. W., and Daemen, T. (2015) Tattoo delivery of a Semliki Forest Virus-based vaccine encoding human papillomavirus E6 and E7, Vaccines (Basel), 3, 221–238, doi: 10.3390/vaccines3020221.
Lundstrom, K. (2019) Plasmid DNA-based alphavirus vaccines, Vaccines, 7, 29, doi: 10.3390/vaccines7010029.
Hsu, K. F., Hung, C. F., Cheng, W. F., He, L., Slater, L. A., Ling, M., and Wu, T. C. (2001) Enhancement of suicidal DNA vaccine potency by linking Mycobacterium tuberculosis heat shock protein 70 to an antigen, Gene Ther., 8, 376–383, doi: 10.1038/sj.gt.3301408.
Kim, T. W., Hung, C. F., Juang, J., He, L., Hardwick, J. M., and Wu, T. C. (2004) Enhancement of suicidal DNA vaccine potency by delaying suicidal DNA-induced cell death, Gene Ther., 11, 336–342, doi: 10.1038/sj.gt.3302164.
Van de Wall, S., Ljungberg, K., Ip, P. P., Boerma, A., Knudsen, M. L., Nijman, H. W., Liljestrom, P., and Daemen, T. (2018) Potent therapeutic efficacy of an alphavirus replicon DNA vaccine expressing human papilloma virus E6 and E7 antigens, Oncoimmunology, 7, e1487913, doi: 10.1080/2162402X.2018.1487913.
Varnavski, A. N., Young, P. R., and Khromykh, A. A. (2000) Stable high-level expression of heterologous genes in vitro and in vivo by noncytopathic DNA-based Kunjin virus replicon vectors, J. Virol., 74, 4394–4403, doi: 10.1128/JVI.74.9.4394-4403.2000.
Herd, K. A., Harvey, T., Khromykh, A. A., and Tindle, R. W. (2004) Recombinant Kunjin virus replicon vaccines induce protective T-cell immunity against human papillomavirus 16 E7-expressing tumour, Virology, 319, 237–248, 10.1016/j.virol.2003.10.032.
Sebastian, M., Papachristofilou, A., Weiss, C., Fruh, M., Cathomas, R., Hilbe, W., Wehler, T., Rippin, G., Koch, S. D., Scheel, B., Fotin- Mleczek, M., Heidenreich, R., Kallen, K. J., Gnad- Vogt, U., and Zippelius, A. (2014) Phase Ib study evaluating a selfadjuvanted mRNA cancer vaccine (RNActive®) combined with local radiation as consolidation and maintenance treatment for patients with stage IV non-small cell lung cancer, BMC Cancer, 14, 748, doi: 10.1186/1471-2407-14-748.
Coleman, H. N., Greenfield, W. W., Stratton, S. L., Vaughn, R., Kieber, A., Moerman-Herzog, A. M., Spencer, H. J., Hitt, W. C., Quick, C. M., Hutchins, L. F., Mackintosh, S. G., Edmondson, R. D., Erickson, S. W., and Nakagawa, M. (2016) Human papillomavirus type 16 viral load is decreased following a therapeutic vaccination, Cancer Immunol. Immunother., 65, 563–573, doi: 10.1007/ s00262-016-1821-x.
Wang, C., Dickie, O., Sutavani, K. M., Pointer, C., Thomas, G. J., and Savelyeva, N. (2018) Targeting head and neck cancer by vaccination, Front. Immunol., 9, 830, doi: 10.3389/fimmu.2018.00830.
Lin, K., Doolan, K., Hung, C. F., and Wu, T. C. (2010) Perspectives for preventive and therapeutic HPV vaccines, J. Formos. Med. Assoc., 109, 4–24, doi: 10.1016/S0929-6646(10)60017-4.
Su, J. H., Wu, A., Scotney, E., Ma, B., Monie, A., Hung, C. F., and Wu, T. C. (2010) Immunotherapy for cervical cancer: research status and clinical potential, BioDrugs, 24, 109–129, doi: 10.2165/11532810-000000000-00000.
Hung, C. F., Ma, B., Monie, A., Tsen, S. W., and Wu, T. C. (2008) Therapeutic human papillomavirus vaccines: current clinical trials and future directions, Expert Opin. Biol. Ther., 8, 421–439, doi: 10.1517/14712598.8.4.421.
Zwaveling, S., Ferreira Mota, S. C., Nouta, J., Johnson, M., Lipford, G. B., Offringa, R., van der Burg, S. H., and Melief, C. J. (2002) Established human papillomavirus type 16 expressing tumors are effectively eradicated following vaccination with long peptides, J. Immunol., 169, 350–358, doi: 10.4049/jimmunol.169.1.350.
De Vos van Steenwijk, P. J., van Poelgeest, M. I., Ramwadhdoebe, T. H., Lowik, M. J., Berends-van der Meer, D. M., van der Minne, C. E., Loof, N. M., Stynenbosch, L. F., Fathers, L. M., Valentijn, A. R., Oostendorp, J., Osse, E. M., Fleuren, G. J., Nooij, L., Kagie, M. J., Hellebrekers, B. W., Melief, C. J., Welters, M. J., van der Burg, S. H., and Kenter, G. G. (2014) The long-term immune response after HPV16 peptide vaccination in women with low-grade premalignant disorders of the uterine cervix: a placebo-controlled phase II study, Cancer Immunol. Immunother., 63, 147–160, doi: 10.1007/s00262-013-1499-2.
Melief, C. J., Gerritsen, W. R., Welters, M., Vergote, I., Kroep, J. R., Kenter, G., Ottevanger, P. B., Tjalma, W. A., Denys, H., Nijman, H., van Poelgeest, M. I. E., Reyners, A. K. L., Velu, T. J., Blumenstein, B. A., Goffin, F., Lalisang, R. I., Stead, R. B., and van der Burg, S. (2017) Correlation between strength of T-cell response against HPV16 and survival after vaccination with HPV16 long peptides in combination with chemotherapy for late-stage cervical cancer, J. Clin. Oncol., 35, 140, doi: 10.1200/ JCO.2017.35.7-suppl.140.
Massarelli, E., William, W., Johnson, F., Kies, M., Ferrarotto, R., Guo, M., Feng, L., Lee, J. J., Tran, H., Kim, Y. U., Haymaker, C., Bernatchez, C., Curran, M., Zecchini Barrese, T., Rodriguez Canales, J., Wistuba, I., Li, L., Wang, J., van der Burg, S. H., Melief, C. J., and Glisson, B. (2019) Combining immune checkpoint blockade and tumor-specific vaccine for patients with incurable human papillomavirus 16-related cancer: a phase 2 clinical trial, JAMA Oncol., 5, 67–73, doi: 10.1001/jamaoncol.2018.4051.
Van der Burg, S. H., Kwappenberg, K. M., O’Neill, T., Brandt, R. M., Melief, C. J., Hickling, J. K., and Offringa, R. (2001) Pre-clinical safety and efficacy of TA-CIN, a recombinant HPV16 L2E6E7 fusion protein vaccine, in homologous and heterologous prime-boost regimens, Vaccine, 19, 3652–3660.
De Jong, A., O’Neill, T., Khan, A. Y., Kwappenberg, K. M., Chisholm, S. E., Whittle, N. R., Dobson, J. A., Jack, L. C., St. Clair Roberts, J. A., Offringa, R., van der Burg, S. H., and Hickling, J. K. (2002) Enhancement of human papillomavirus (HPV) type 16 E6 and E7-specific T-cell immunity in healthy volunteers through vaccination with TA-CIN, an HPV16 L2E7E6 fusion protein vaccine, Vaccine, 20, 3456–3464.
Daayana, S., Elkord, E., Winters, U., Pawlita, M., Roden, R., Stern, P. L., and Kitchener, H. C. (2010) Phase II trial of imiquimod and HPV therapeutic vaccination in patients with vulval intraepithelial neoplasia, Br. J. Cancer, 102, 1129–1136, doi: 10.1038/sj.bjc.6605611.
Hibbitts, S. (2010) TA-CIN, a vaccine incorporating a recombinant HPV fusion protein (HPV16 L2E6E7) for the potential treatment of HPV16-associated genital diseases, Curr. Opin. Mol. Ther., 12, 598–606.
Van Damme, P., Bouillette-Marussig, M., Hens, A., De Coster, I., Depuydt, C., Goubier, A., Van Tendeloo, V., Cools, N., Goossens, H., Hercend, T., Timmerman, B., and Bissery, M. C. (2016) GTL001, a therapeutic vaccine for women infected with human papillomavirus 16 or 18 and normal cervical cytology: results of a phase I clinical trial, Clin. Cancer Res., 22, 3238–3248, doi: 10.1158/1078-0432.CCR-16-0085.
Granadillo, M., Vallespi, M. G., Batte, A., Mendoza, O., Soria, Y., Lugo, V. M., and Torrens, I. (2011) A novel fusion protein-based vaccine comprising a cell penetrating and immunostimulatory peptide linked to human papillomavirus (HPV) type 16 E7 antigen generates potent immunologic and anti-tumor responses in mice, Vaccine, 29, 920–930, doi: 10.1016/j.vaccine.2010.11.083.
Ferraro, B., Morrow, M. P., Hutnick, N. A., Shin, T. H., Lucke, C. E., and Weiner, D. B. (2011) Clinical applications of DNA vaccines: current progress, Clin. Infect. Dis., 53, 296–302, doi: 10.1093/cid/cir334.
Maldonado, L., Teague, J. E., Morrow, M. P., Jotova, I., Wu, T. C., Wang, C., Desmarais, C., Boyer, J. D., Tycko, B., Robins, H. S., Clark, R. A., and Trimble, C. L. (2014) Intramuscular therapeutic vaccination targeting HPV16 induces T-cell responses that localize in mucosal lesions, Sci. Transl. Med., 6, 221ra13, doi: 10.1126/scitranslmed. 3007323.
Trimble, C., Lin, C. T., Hung, C. F., Pai, S., Juang, J., He, L., Gillison, M., Pardoll, D., Wu, L., and Wu, T. C. (2003) Comparison of the CD8+ T-cell responses and antitumor effects generated by DNA vaccine administered through gene gun, biojector, and syringe, Vaccine, 21, 4036–4042.
Alvarez, R. D., Huh, W. K., Bae, S., Lamb, L. S., Jr., Conner, M. G., Boyer, J., Wang, C., Hung, C. F., Sauter, E., Paradis, M., Adams, E. A., Hester, S., Jackson, B. E., Wu, T. C., and Trimble, C. L. (2016) A pilot study of pNGVL4a-CRT/E7(detox) for the treatment of patients with HPV16+ cervical intraepithelial neoplasia 2/3 (CIN2/3), Gynecol. Oncol., 140, 245–252, doi: 10.1016/ j.ygyno.2015.11.026.
Chandra, J., Dutton, J. L., Li, B., Woo, W. P., Xu, Y., Tolley, L. K., Yong, M., Wells, J. W., Leggatt, G. R., Finlayson, N., and Frazer, I. H. (2017) DNA vaccine encoding HPV16 oncogenes E6 and E7 induces potent cell-mediated and humoral immunity which protects in tumor challenge and drives E7-expressing skin graft rejection, J. Immunother., 40, 62–70, doi: 10.1097/CJI.0000000000000156.
Lu, S., Wang, S., and Grimes-Serrano, J. M. (2008) Current progress of DNA vaccine studies in humans, Expert Rev. Vaccines, 7, 175–191, doi: 10.1586/14760584.7.2.175.
Ali, A. A., McCrudden, C. M., McCaffrey, J., McBride, J. W., Cole, G., Dunne, N. J., Robson, T., Kissenpfennig, A., Donnelly, R. F., and McCarthy, H. O. (2017) DNA vaccination for cervical cancer; a novel technology platform of RALA mediated gene delivery via polymeric microneedles, Nanomedicine, 13, 921–932, doi: 10.1016/j.nano.2016.11.019.85.
Samuels, S., Marijne Heeren, A., Zijlmans, H. J. M. A. A., Welters, M. J. P., van den Berg, J. H., Philips, D., Kvistborg, P., Ehsan, I., Scholl, S. M. E., Nuijen, B., Schumacher, T. N. M., van Beurden, M., Jordanova, E. S., Haanen, J. B. A. G., van der Burg, S. H., and Kenter, G. G. (2017) HPV16 E7 DNA tattooing: safety, immunogenicity, and clinical response in patients with HPV-positive vulvar intraepithelial neoplasia, Cancer Immunol. Immunother., 66, 1163–1173, doi: 10.1007/s00262-017-2006-y.
Ostor, A. G. (1993) Natural history of cervical intraepithelial neoplasia—a critical review, Int. J. Gynecol. Pathol., 12, 186–192.
Morrow, M. P., Kraynyak, K. A., Sylvester, A. J., Dallas, M., Knoblock, D., Boyer, J. D., Yan, J., Vang, R., Khan, A. S., Humeau, L., Sardesai, N. Y., Kim, J. J., Plotkin, S., Weiner, D. B., Trimble, C. L., and Bagarazzi, M. L. (2018) Clinical and immunologic biomarkers for histologic regression of high-grade cervical dysplasia and clearance of HPV16 and HPV18 after immunotherapy, Clin. Cancer Res., 24, 276–294, doi: 10.1158/1078-0432.CCR-17-2335.
Santos, P. M., and Butterfield, L. H. (2018) Dendritic cellbased cancer vaccines, J. Immunol., 200, 443–449, doi: 10.4049/jimmunol.1701024.
Santin, A. D., Bellone, S., Roman, J. J., Burnett, A., Cannon, M. J., and Pecorelli, S. (2005) Therapeutic vaccines for cervical cancer: dendritic cell-based immunotherapy, Curr. Pharm. Des., 11, 3485–3500.
Ahn, Y. H., Hong, S. O., Kim, J. H., Noh, K. H., Song, K. H., Lee, Y. H., Jeon, J. H., Kim, D. W., Seo, J. H., and Kim, T. W. (2015) The siRNA cocktail targeting interleukin 10 receptor and transforming growth factor-beta receptor on dendritic cells potentiates tumour antigen-specific CD8(+) T-cell immunity, Clin. Exp. Immunol., 181, 164–178, doi: 10.1111/cei.12620.
Santin, A. D., Bellone, S., Palmieri, M., Zanolini, A., Ravaggi, A., Siegel, E. R., Roman, J. J., Pecorelli, S., and Cannon, M. J. (2007) Human papillomavirus type 16 and 18 E7-pulsed dendritic cell vaccination of stage IB or IIA cervical cancer patients: a phase I escalating-dose trial, J. Virol., 82, 1968–1979, doi: 10.1128/JVI.02343-07.
Brun, J. L., Rajaonarison, J., Nocart, N., Hoarau, L., Brun, S., and Garrigue, I. (2018) Targeted immunotherapy of high-grade cervical intra-epithelial neoplasia: expectations from clinical trials, Mol. Clin. Oncol., 8, 227–235, doi: 10.3892/mco.2017.1531.
Mikyskova, R., Indrova, M., Simova, J., Jandlova, T., Bieblova, J., Jinoch, P., Bubenik, J., and Vonka, V. (2004) Treatment of minimal residual disease after surgery or chemotherapy in mice carrying HPV16-associated tumours: cytokine and gene therapy with IL-2 and GMCSF, Int. J. Oncol., 24, 161–167, doi: 10.3892/ijo.24.1.161.
Chang, E. Y., Chen, C. H., Ji, H., Wang, T. L., Hung, K., Lee, B. P., Huang, A. Y., Kurman, R. J., Pardoll, D. M., and Wu, T. (2000) Antigen-specific cancer immunotherapy using a GM-CSF secreting allogeneic tumor cell-based vaccine, Int. J. Cancer, 86, 725–730.
Schneider, K., Gronhoj, C., Hahn, C. H., and von Buchwald, C. (2018) Therapeutic human papillomavirus vaccines in head and neck cancer: a systematic review of current clinical trials, Vaccine, 36, 6594–6605, doi: 10.1016/j.vaccine.2018.09.027.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Russian Text © The Author(s), 2019, published in Biokhimiya, 2019, Vol. 84, No. 7, pp. 1016–1035.
Rights and permissions
About this article
Cite this article
Vonsky, M.S., Runov, A.L., Gordeychuk, I.V. et al. Therapeutic Vaccines Against Human Papilloma Viruses: Achievements and Prospects. Biochemistry Moscow 84, 800–816 (2019). https://doi.org/10.1134/S0006297919070101
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297919070101