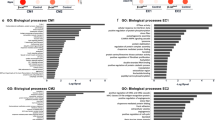
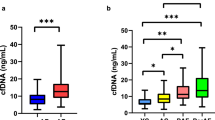
Abstract
Differential diagnosis of arrhythmogenic cardiomyopathy (ACM) during the disease latent phase is a challenging clinical problem that requires identification of early ACM biomarkers. Because extracellular nucleic acids are stable, specific, and can be easily detected, they can be used as reliable biomarkers of various diseases. In this study, we analyzed the levels of extracellular microRNAs and mitochondrial DNA in the conditioned medium collected from cardiomyocytes differentiated from induced pluripotent stem cells of ACM patients and healthy donor. Several microRNAs were expressed differently by the affected and healthy cardiomyocytes; therefore, they could be considered as potential ACM biomarkers.
Similar content being viewed by others
Abbreviations
- ACM:
-
arrhythmogenic cardiomyopathy
- DAPI:
-
4′,6-diamidino-2-phenylindole
- iPSC:
-
induced pluripotent stem cell
- miR:
-
microRNA (miRNA)
- mtDNA:
-
mitochondrial DNA
- PBS:
-
phosphate buffered saline
References
Thiene, G., Nava, A., Corrado, D., Rossi, L., and Pennelli, N. (1988) Right ventricular cardiomyopathy and sudden death in young people, N. Engl. J. Med., 318, 129–133.
Lazzarini, E., Jongbloed, J. D. H., Pilichou, K., Thiene, G., Basso, C., Bikker, H., Charbon, B., Swertz, M., van Tintelen, J. P., and van der Zwaag, P. A. (2015) The ARVD/C genetic variants database: 2014 update, Hum. Mutat., 36, 403–410.
Corrado, D., Basso, C., and Thiene, G. (2009) Arrhythmogenic right ventricular cardiomyopathy: an update, Heart, 95, 766–773.
Bartel, D. P. (2004) MicroRNAs: genomics, biogenesis, mechanism, and function, Cell, 116, 281–297.
He, L., and Hannon, G. J. (2004) MicroRNAs: small RNAs with a big role in gene regulation, Nat. Rev. Genet., 5, 522–531.
Sohel, M. H. (2016) Extracellular/circulating microRNAs: release mechanisms, functions and challenges, Achiev. Life Sci., 10, 175–186.
Arroyo, J. D., Chevillet, J. R., Kroh, E. M., Ruf, I. K., Pritchard, C. C., Gibson, D. F., Mitchell, P. S., Bennett, C. F., Pogosova–Agadjanyan, E. L., Stirewalt, D. L., Tait, J. F., and Tewari, M. (2011) Argonaute 2 complexes carry a pop–ulation of circulating microRNAs independent of vesicles in human plasma, Proc. Natl. Acad. Sci. USA, 108, 5003–5008.
Turchinovich, A., Weiz, L., Langheinz, A., and Burwinkel, B. (2011) Characterization of extracellular circulating microRNA, Nucleic Acids Res., 39, 7223–7233.
Vickers, K. C., Palmisano, B. T., Shoucri, B. M., Shamburek, R. D., and Remaley, A. T. (2011) MicroRNAs are transported in plasma and delivered to recipient cells by high–density lipoproteins, Nat. Cell Biol., 13, 423–433.
Wagner, J., Riwanto, M., Besler, C., Knau, A., Fichtlscherer, S., Roxe, T., Zeiher, A. M., Landmesser, U., and Dimmeler, S. (2013) Characterization of levels and cel–lular transfer of circulating lipoprotein–bound microRNAs, Arterioscler. Thromb. Vasc. Biol., 33, 1392–1400.
Sommariva, E., D’Alessandra, Y., Farina, F. M., Casella, M., Cattaneo, F., Catto, V., Chiesa, M., Stadiotti, I., Brambilla, S., Dello Russo, A., Carbucicchio, C., Vettor, G., Riggio, D., Sandri, M. T., Barbuti, A., Vernillo, G., Muratori, M., Dal Ferro, M., Sinagra, G., Moimas, S., Giacca, M., Colombo, G. I., Pompilio, G., and Tondo, C. (2017) MiRNA–320a as a potential novel circulating bio–marker of arrhythmogenic cardiomyopathy, Sci. Rep., 7, 4802, doi: 10.1038/s41598–017–05001–z.
Zhang, H., Liu, S., Dong, T., Yang, J., Xie, Y., Wu, Y., Kang, K., Hu, S., Gou, D., and Wei, Y. (2016) Profiling of differentially expressed microRNAs in arrhythmogenic right ventricular cardiomyopathy, Sci. Rep., 6, 28101, doi: 10.1038/srep28101.
Sun, J.–Y., Huang, Y., Li, J.–P., Zhang, X., Wang, L., Meng, Y.–L., Yan, B., Bian, Y.–Q., Zhao, J., Wang, W.–Z., Yang, A.–G., and Zhang, R. (2012) MicroRNA–320a sup–presses human colon cancer cell proliferation by directly targeting beta–catenin, Biochem. Biophys. Res. Commun., 420, 787–792.
Huang, K., Zhang, J.–X., Han, L., You, Y.–P., Jiang, T., Pu, P.–Y., and Kang, C.–S. (2010) MicroRNA roles in beta–catenin pathway, Mol. Cancer, 9, 252.
Hashimi, S. T., Fulcher, J. A., Chang, M. H., Gov, L., Wang, S., and Lee, B. (2009) MicroRNA profiling identi–fies miR–34a and miR–21 and their target genes JAG1 and WNT1 in the coordinate regulation of dendritic cell differ–entiation, Blood, 114, 404–414.
Lin, C.–W., Chang, Y.–L., Chang, Y.–C., Lin, J.–C., Chen, C.–C., Pan, S.–H., Wu, C.–T., Chen, H.–Y., Yang, S.–C., Hong, T.–M., and Yang, P.–C. (2013) MicroRNA–135b pro–motes lung cancer metastasis by regulating multiple targets in the Hippo pathway and LZTS1, Nat. Commun., 4, 1877.
Yamada, S., Hsiao, Y.–W., Chang, S.–L., Lin, Y.–J., Lo, L.–W., Chung, F.–P., Chiang, S.–J., Hu, Y.–F., Tuan, T.–C., Chao, T.–F., Liao, J.–N., Lin, C.–Y., Chang, Y.–T., Te, A. L. D., Tsai, Y.–N., and Chen, S.–A. (2018) Circulating microRNAs in arrhythmogenic right ventricular cardiomyo–pathy with ventricular arrhythmia, Europace, 20, f37–f45.
Sagan, L. (1967) On the origin of mitosing cells, J. Theor. Biol., 14, 255–274.
Gray, M. W., Burger, G., and Lang, B. F. (2001) The origin and early evolution of mitochondria, Genome Biol., 2, doi: 10.1186/gb–2001–2–6–reviews1018.
Zhang, Q., Raoof, M., Chen, Y., Sumi, Y., Sursal, T., Junger, W., Brohi, K., Itagaki, K., and Hauser, C. J. (2010) Circulating mitochondrial DAMPs cause inflammatory responses to injury, Nature, 464, 104–107.
Chiu, R. W. K., Chan, L. Y. S., Lam, N. Y. L., Tsui, N. B. Y., Ng, E. K. O., Rainer, T. H., and Lo, Y. M. D. (2003) Quantitative analysis of circulating mitochondrial DNA in plasma, Clin. Chem., 49, 719–726.
Bliksoen, M., Mariero, L. H., Ohm, I. K., Haugen, F., Yndestad, A., Solheim, S., Seljeflot, I., Ranheim, T., Andersen, G. O., Aukrust, P., Valen, G., and Vinge, L. E. (2012) Increased circulating mitochondrial DNA after myocardial infarction, Int. J. Cardiol., 158, 132–134.
Sudakov, N. P., Apartsin, K. A., Lepekhova, S. A., Nikiforov, S. B., Katyshev, A. I., Lifshits, G. I., Vybivantseva, A. V., and Konstantinov, Y. M. (2017) The level of free circulating mitochondrial DNA in blood as predictor of death in case of acute coronary syndrome, Eur. J. Med. Res., 22, 1.
Oka, T., Hikoso, S., Yamaguchi, O., Taneike, M., Takeda, T., Tamai, T., Oyabu, J., Murakawa, T., Nakayama, H., Nishida, K., Akira, S., Yamamoto, A., Komuro, I., and Otsu, K. (2012) Mitochondrial DNA that escapes from autophagy causes inflammation and heart failure, Nature, 485, 251–255.
Ye, W., Tang, X., Yang, Z., Liu, C., Zhang, X., Jin, J., and Lyu, J. (2017) Plasma–derived exosomes contribute to inflammation via the TLR9–NF–kappaB pathway in chron–ic heart failure patients, Mol. Immunol., 87, 114–121.
Khudiakov, A. A., Kostina, D. A., Kostareva, A. A., Tomilin, A. N., and Malashicheva, A. B. (2015) Influence of mutations in the plakophilin–2 gene on the activity of the canonic signaling pathway Wnt, Tsitologiya, 57, 868–875.
Khudiakov, A., Kostina, D., Zlotina, A., Yany, N., Sergushichev, A., Pervunina, T., Tomilin, A., Kostareva, A., and Malashicheva, A. (2017) Generation of iPSC line from patient with arrhythmogenic right ventricular cardiomy–opathy carrying mutations in PKP2 gene, Stem Cell Res., 24, 85–88.
Burridge, P. W., Matsa, E., Shukla, P., Lin, Z. C., Churko, J. M., Ebert, A. D., Lan, F., Diecke, S., Huber, B., Mordwinkin, N. M., Plews, J. R., Abilez, O. J., Cui, B., Gold, J. D., and Wu, J. C. (2014) Chemically defined gener–ation of human cardiomyocytes, Nat. Methods, 11, 855–860.
Rothfuss, O., Gasser, T., and Patenge, N. (2010) Analysis of differential DNA damage in the mitochondrial genome employing a semi–long run real–time PCR approach, Nucleic Acids Res., 38, e24.
Chen, X., Ba, Y., Ma, L., Cai, X., Yin, Y., Wang, K., Guo, J., Zhang, Y., Chen, J., Guo, X., Li, Q., Li, X., Wang, W., Zhang, Y., Wang, J., Jiang, X., Xiang, Y., Xu, C., Zheng, P., Zhang, J., Li, R., Zhang, H., Shang, X., Gong, T., Ning, G., Wang, J., Zen, K., Zhang, J., and Zhang, C.–Y. (2008) Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases, Cell Res., 18, 997–1006.
Gupta, S. K., Bang, C., and Thum, T. (2010) Circulating microRNAs as biomarkers and potential paracrine media–tors of cardiovascular disease, Circ. Cardiovasc. Genet., 3, 484–488.
Wang, K., Zhang, S., Marzolf, B., Troisch, P., Brightman, A., Hu, Z., Hood, L. E., and Galas, D. J. (2009) Circulating microRNAs, potential biomarkers for drug–induced liver injury, Proc. Natl. Acad. Sci. USA, 106, 4402–4407.
Casini, S., Verkerk, A. O., and Remme, C. A. (2017) Human iPSC–derived cardiomyocytes for investigation of disease mechanisms and therapeutic strategies in inherited arrhythmia syndromes: strengths and limitations, Cardiovasc. Drugs Ther., 31, 325–344.
Fu, J.–D., Rushing, S. N., Lieu, D. K., Chan, C. W., Kong, C.–W., Geng, L., Wilson, K. D., Chiamvimonvat, N., Boheler, K. R., Wu, J. C., Keller, G., Hajjar, R. J., and Li, R. A. (2011) Distinct roles of microRNA–1 and–499 in ventricular specification and functional maturation of human embryonic stem cell–derived cardiomyocytes, PLoS One, 6, e27417.
Dubash, A. D., Kam, C. Y., Aguado, B. A., Patel, D. M., Delmar, M., Shea, L. D., and Green, K. J. (2016) Plakophilin–2 loss promotes TGF–β1/p38 MAPK–depend–ent fibrotic gene expression in cardiomyocytes, J. Cell Biol., 212, 425–438.
Chen, S. N., Gurha, P., Lombardi, R., Ruggiero, A., Willerson, J. T., and Marian, A. J. (2014) The Hippo path–way is activated and is a causal mechanism for adipogenesis in arrhythmogenic cardiomyopathy, Circ. Res., 114, 454–468.
Kim, C., Wong, J., Wen, J., Wang, S., Wang, C., Spiering, S., Kan, N. G., Forcales, S., Puri, P. L., Leone, T. C., Marine, J. E., Calkins, H., Kelly, D. P., Judge, D. P., and Chen, H.–S. V. (2013) Studying arrhythmogenic right ven–tricular dysplasia with patient–specific iPSCs, Nature, 494, 105–110.
Sommariva, E., Brambilla, S., Carbucicchio, C., Gambini, E., Meraviglia, V., Dello Russo, A., Farina, F. M., Casella, M., Catto, V., Pontone, G., Chiesa, M., Stadiotti, I., Cogliati, E., Paolin, A., Ouali Alami, N., Preziuso, C., D’Amati, G., Colombo, G. I., Rossini, A., Capogrossi, M. C., Tondo, C., and Pompilio, G. (2016) Cardiac mes–enchymal stromal cells are a source of adipocytes in arrhythmogenic cardiomyopathy, Eur. Heart J., 37, 1835–1846.
Kuwabara, Y., Ono, K., Horie, T., Nishi, H., Nagao, K., Kinoshita, M., Watanabe, S., Baba, O., Kojima, Y., Shizuta, S., Imai, M., Tamura, T., Kita, T., and Kimura, T. (2011) Increased microRNA–1 and microRNA–133a levels in serum of patients with cardiovascular disease indicate myocardial damage, Circ. Cardiovasc. Genet., 4, 446–454.
Thum, T., Gross, C., Fiedler, J., Fischer, T., Kissler, S., Bussen, M., Galuppo, P., Just, S., Rottbauer, W., Frantz, S., Castoldi, M., Soutschek, J., Koteliansky, V., Rosenwald, A., Basson, M. A., Licht, J. D., Pena, J. T. R., Rouhanifard, S. H., Muckenthaler, M. U., Tuschl, T., Martin, G. R., Bauersachs, J., and Engelhardt, S. (2008) MicroRNA–21 contributes to myocardial disease by stimu–lating MAP kinase signalling in fibroblasts, Nature, 456, 980–984.
Roy, S., Khanna, S., Hussain, S.–R. A., Biswas, S., Azad, A., Rink, C., Gnyawali, S., Shilo, S., Nuovo, G. J., and Sen, C. K. (2009) MicroRNA expression in response to murine myocardial infarction: miR–21 regulates fibroblast metalloprotease–2 via phosphatase and tensin homologue, Cardiovasc. Res., 82, 21–29.
Van Rooij, E., Sutherland, L. B., Thatcher, J. E., DiMaio, J. M., Naseem, R. H., Marshall, W. S., Hill, J. A., and Olson, E. N. (2008) Dysregulation of microRNAs after myocardial infarction reveals a role of miR–29 in cardiac fibrosis, Proc. Natl. Acad. Sci. USA, 105, 13027–13032.
Matkovich, S. J., Wang, W., Tu, Y., Eschenbacher, W. H., Dorn, L. E., Condorelli, G., Diwan, A., Nerbonne, J. M., and Dorn, G. W. (2010) MicroRNA–133a protects against myocardial fibrosis and modulates electrical repolarization without affecting hypertrophy in pressure–overloaded adult hearts, Circ. Res., 106, 166–175.
Gerin, I., Bommer, G. T., McCoin, C. S., Sousa, K. M., Krishnan, V., and MacDougald, O. A. (2010) Roles for miRNANA–378/378* in adipocyte gene expression and lipogenesis, Am. J. Physiol. Endocrinol. Metab., 299, E198–206.
Beltrami, C., Besnier, M., Shantikumar, S., Shearn, A. I. U., Rajakaruna, C., Laftah, A., Sessa, F., Spinetti, G., Petretto, E., Angelini, G. D., and Emanueli, C. (2017) Human pericardial fluid contains exosomes enriched with cardiovascular–expressed microRNAs and promotes thera–peutic angiogenesis, Mol. Ther., 25, 679–693.
Author information
Authors and Affiliations
Corresponding author
Additional information
Russian Text © A. A. Khudiakov, N. A. Smolina, K. I. Perepelina, A. B. Malashicheva, A. A. Kostareva, 2019, published in Biokhimiya, 2019, Vol. 84, No. 3, pp. 392–403.
Rights and permissions
About this article
Cite this article
Khudiakov, A.A., Smolina, N.A., Perepelina, K.I. et al. Extracellular MicroRNAs and Mitochondrial DNA as Potential Biomarkers of Arrhythmogenic Cardiomyopathy. Biochemistry Moscow 84, 272–282 (2019). https://doi.org/10.1134/S000629791903009X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S000629791903009X