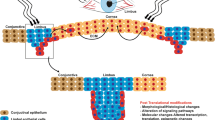
Abstract
Visual system is at high risk of iatrogenic damage. Laser ocular surgery, the use of powerful illumination devices in diagnostics and surgical treatment of eye diseases, as well as long surgeries under general anesthesia provoke the development of chronic degenerative changes in eye tissues, primarily in the cornea and the retina. Despite the existence of approaches for prevention and treatment of these complications, the efficacy of these approaches is often limited. Here, we review the mechanisms of iatrogenic damage to eye tissues at the cellular and biochemical levels. It is well recognized that oxidative stress is one of the main factors hindering regeneration of eye tissues after injuries and, thereby, aggravating iatrogenic eye disorders. It is accompanied by the downregulation of low–molecular–weight antioxidants and antioxidant enzymes, as well as changes in the expression and redox status of proteins in the damaged tissue. In this regard, antioxidant therapy, in particular, the use of highly effective mitochondria–targeted antioxidants such as SkQ1, is considered as a promising approach to the prevention of iatrogenesis. Recent findings indicate that the most efficient protection of eye tissues from the iatrogenic injury is achieved by preventive use of these antioxidants. In addition to preventing corneal and retinal cell death induced by oxidative stress, SkQ1 contributes to the restoration of innate antioxidant defense of these tissues and suppresses local inflammatory response. Since the timing of routine medical manipulations is usually known in advance, iatrogenic damage to the ocular tissues can be successfully prevented using mitochondria–targeted therapy.
Similar content being viewed by others
Abbreviations
- PCRD:
-
photochemical retinal damage
- PDES:
-
perioperative dry eye syndrome
- ROS:
-
reactive oxygen species
- RPE:
-
retinal pigment epithelium
References
Gomes, J. A. P., Azar, D. T., Baudouin, C., Efron, N., Hirayama, M., Horwath–Winter, J., Kim, T., Mehta, J. S., Messmer, E. M., Pepose, J. S., Sangwan, V. S., Weiner, A. L., Wilson, S. E., and Wolffsohn, J. S. (2017) TFOS DEWS II iatrogenic report, Ocul. Surf., 15, 511–538.
Malafa, M. M., Coleman, J. E., Bowman, R. W., and Rohrich, R. J. (2016) Perioperative corneal abrasion: updated guidelines for prevention and management, Plast. Reconstr. Surg., 137, 790e–798e.
Wolffe, M. (2016) How safe is the light during ophthalmic diagnosis and surgery, Eye (Lond.), 30, 186–188.
Iomdina, E. N., Tarutta, E. P., Ignat’eva, N. Yu., Kostanyan, I. A., Minkevich, N. I., Shehter, A. B., Danilov, N. A., Kvaatsheliya, N. G., and Cherhisheva, S. G. (2008) Current achievements in basic studies of the pathogenesis of progressing myopia, Ross. Oftalmol. Zh., 1, 7–12.
Batra, Y. K., and Bali, I. M. (1977) Corneal abrasions during general anesthesia, Anesth. Analg., 56, 363–365.
Glickman, R. D. (2002) Phototoxicity to the retina: mechanisms of damage, Int. J. Toxicol., 21, 473–490.
Zernii, E. Y., Baksheeva, V. E., Iomdina, E. N., Averina, O. A., Permyakov, S. E., Philippov, P. P., Zamyatnin, A. A., and Senin, I. I. (2016) Rabbit models of ocular diseases: new relevance for classical approaches, CNS Neurol. Disord. Drug Targets, 15, 267–291.
Buddi, R., Lin, B., Atilano, S. R., Zorapapel, N. C., Kenney, M. C., and Brown, D. J. (2002) Evidence of oxidative stress in human corneal diseases, J. Histochem. Cytochem., 50, 341–351.
Moreno, M. C., Campanelli, J., Sande, P., Saenz, D. A., Sarmiento, M. I. K., and Rosenstein, R. E. (2004) Retinal oxidative stress induced by high intraocular pressure, Free Radic. Biol. Med., 37, 803–812.
Gandhi, S., and Jain, S. (2014) The Anatomy and Physiology of Cornea, Keratoprostheses and Artificial Corneas: Fundamentals and Surgical Applications, Springer, Berlin–Heidelberg, pp. 19–25.
Kolozsvari, L., Nogradi, A., Hopp, B., and Bor, Z. (2002) UV absorbance of the human cornea in the 240–to 400–nm range, Invest. Ophthalmol. Vis. Sci., 43, 2165–2168.
Chen, Y., Mehta, G., and Vasiliou, V. (2009) Antioxidant defenses in the ocular surface, Ocul. Surf., 7, 176–185.
Fini, M. (1999) Keratocyte and fibroblast phenotypes in the repairing cornea, Prog. Retin. Eye Res., 18, 529–551.
Zhang, W., Li, H., Ogando, D. G., Li, S., Feng, M., Price, F. W., Jr., Tennessen, J. M., and Bonanno, J. A. (2017) Glutaminolysis is essential for energy production and ion transport in human corneal endothelium, EBioMedicine, 16, 292–301.
Kim, K. M., Shin, Y.–T., and Kim, H. K. (2012) Effect of autologous platelet–rich plasma on persistent corneal epithelial defect after infectious keratitis, Jpn. J. Ophthalmol., 56, 544–550.
Baudouin, C. (2001) The pathology of dry eye, Surv. Ophthalmol., 45, S211–S220.
Zernii, E. Yu., Golovastova, M. O., Baksheeva, V. E., Kabanova, E. I., Ishutina, I. E., Gancharova, O. S., Gusev, A. E., Savchenko, M. S., Loboda, A. P., Sotnikova, L. F., Zamyatnin, A. A., Jr., Philippov, P. P., and Senin, I. I. (2016) Alterations in tear biochemistry associated with chronic dry eye syndrome in postanesthetic period, Biochemistry (Moscow), 81, 1549–1557.
Zernii, E. Yu., Baksheeva, V. E., Kabanova, E. I., Tulina, V. V., Golovastova, M. O., Gancharova, O. S., Savchenko, M. S., Sotikova, L. F., Zamyatnin, A. A., Jr., Filippov, P. P., and Senin, I. I. (2018) Effect of general anesthesia duration on recovery of secretion and biochemical properties of tear fluid in the post–anesthetic period, Bull. Exp. Biol. Med., 165, 269–271.
Yu, H.–D., Chou, A.–H., Yang, M.–W., and Chang, C.–J. (2010) An analysis of perioperative eye injuries after nonocular surgery, Acta Anaesthesiol. Taiwan, 48, 122–129.
Orlin, S. E., Kurata, F. K., Krupin, T., Schneider, M., and Glendrange, R. R. (1989) Ocular lubricants and corneal injury during anesthesia, Anesth. Analg., 69, 384–385.
Zeev, M. S.–B., Miller, D. D., and Latkany, R. (2014) Diagnosis of dry eye disease and emerging technologies, Clin. Ophthalmol., 8, 581–590.
Grover, V. K., Kumar, K. V. W., Sharma, S., Sethi, N., and Grewal, S. P. S. (1999) Comparison of methods of eye protection under general anesthesia, Survey Anesthesiol., 43, 75–76.
Wolkoff, P., Nojgaard, J. K., Troiano, P., and Piccoli, B. (2005) Eye complaints in the office environment: precorneal tear film integrity influenced by eye blinking efficiency, Occup. Environ. Med., 62, 4–12.
Mastropasqua, L., Ciancaglini, M., Di Tano, G., Carpineto, P., Lobefalo, L., Loffredo, B., Bosco, D., Columbaro, M., and Falcieri, E. (1998) Ultrastructural changes in rat cornea after prolonged hypobaric hypoxia, J. Submicrosc. Cytol. Pathol., 30, 285–293.
Zernii, E. Yu., Gancharova, O. S., Ishytina, I. E., Baksheeva, V. E., Golovastova, M. O., Kabanova, E. I., Savchenko, M. S., Serebryakova, M. V., Sotikova, L. F., Zamyatnin, A. A., Jr., Filippov, P. P., and Senin, I. I. (2017) Mechanisms of perioperative corneal abrasions: alterations in the tear film proteome, Biochemistry (Moscow) Suppl. Ser. B: Biomed. Chem., 11, 186–193.
Fullard, R. J., and Snyder, C. (1990) Protein levels in non–stimulated and stimulated tears of normal human subjects, Invest. Ophthalmol. Vis. Sci., 31, 1119–1126.
Seitz, B., Rozsival, P., Feuermannova, A., Langenbucher, A., and Naumann, G. O. H. (2003) Penetrating keratoplasty for iatrogenic keratoconus after repeat myopic laser in situ keratomileusis: histologic findings and literature review, J. Cataract Refract. Surg., 29, 2217–2224.
Wang, L., Moss, H., Ventura, B. V., Padilha, H., Hester, C., and Koch, D. D. (2013) Advances in refractive surgery, Asia Pac. J. Ophthalmol. (Phila), 2, 317–327.
Levitt, A. E., Galor, A., Weiss, J. S., Felix, E. R., Martin, E. R., Patin, D. J., Sarantopoulos, K. D., and Levitt, R. C. (2015) Chronic dry eye symptoms after lasik: parallels and lessons to be learned from other persistent post–operative pain disorders, Mol. Pain, 11,21.
Cejkova, J., Stipek, S., Crkovska, J., Ardan, T., Platenik, J., Cejka, C., and Midelfart, A. (2004) UV rays, the prooxidant/antioxidant imbalance in the cornea and oxidative eye damage, Physiol. Res., 53, 1–10.
Leonardi, A., Tavolato, M., Curnow, S. J., Fregona, I. A., Violato, D., and Alio, J. L. (2009) Cytokine and chemokine levels in tears and in corneal fibroblast cultures before and after excimer laser treatment, J. Cataract Refract. Surg., 35, 240–247.
Kochevar, I. E. (1989) Cytotoxicity and mutagenicity of excimer laser radiation, Lasers Surg. Med., 9, 440–445.
Bilgihan, K., Bilgihan, A., Adiguzel, U., Sezer, C., Yis, O., Akyol, G., and Hasanreisoglu, B. (2002) Keratocyte apoptosis and corneal antioxidant enzyme activities after refractive corneal surgery, Eye, 16, 63–68.
Riley, M. V., Susan, S., Peters, M. I., and Schwartz, C. A. (1987) The effects of UV–B irradiation on the corneal endothelium, Curr. Eye Res., 6, 1021–1033.
Carubelli, R., Nordquist, R. E., and Rowsey, J. J. (1990) Role of active oxygen species in corneal ulceration. Effect of hydrogen peroxide generated in situ, Cornea, 9, 161–169.
Ng, S. K., Wood, J. P., Chidlow, G., Han, G., Kittipassorn, T., Peet, D. J., and Casson, R. J. (2015) Cancer–like metabolism of the mammalian retina, Clin. Exp. Ophthalmol., 43, 367–376.
Fletcher, A. E. (2008) Sunlight exposure, antioxidants, and age–related macular degeneration, Arch. Ophthalmol., 126, 1396–1403.
Winkler, B. S. (2008) An hypothesis to account for the renewal of outer segments in rod and cone photoreceptor cells: renewal as a surrogate antioxidant, Invest. Ophthalmol. Vis. Sci., 49, 3259–3261.
Wu, J., Seregard, S., and Algvere, P. V. (2006) Photochemical damage of the retina, Surv. Ophthalmol., 51, 461–481.
Van den Biesen, P. R., Berenschot, T., Verdaasdonk, R. M., van Weelden, H., and van Norren, D. (2000) Endoillumination during vitrectomy and phototoxicity thresholds, Br. J. Ophthalmol., 84, 1372–1375.
Kuhn, F., Morris, R., and Massey, M. (1991) Photic retinal injury from endoillumination during vitrectomy, Am. J. Ophthalmol., 111, 42–46.
McDonald, H. R., and Irvine, A. R. (1983) Light–induced maculopathy from the operating microscope in extracapsular cataract extraction and intraocular lens implantation, Ophthalmology, 90, 945–951.
Michels, M., and Sternberg, P., Jr. (1990) Operating micro–scope–induced retinal phototoxicity: pathophysiology, clinical manifestations and prevention, Surv. Ophthalmol., 34, 237–252.
Postel, E. A., Pulido, J. S., Byrnes, G. A., Heier, J., Waterhouse, W., Han, D. P., Mieler, W. F., Guse, C., and Wipplinger, W. (1998) Long–term follow–up of iatrogenic phototoxicity, Arch. Ophthalmol., 116, 753–757.
Tso, M. O., Fine, B. S., and Zimmerman, L. E. (1972) Photic maculopathy produced by the indirect ophthalmo–scope. 1. Clinical and histopathologic study, Am. J. Ophthalmol., 73, 686–699.
Tso, M. O., and Woodford, B. J. (1983) Effect of photic injury on the retinal tissues, Ophthalmology, 90, 952–963.
Delori, F. C., Webb, R. H., and Sliney, D. H. (2007) Maximum permissible exposures for ocular safety (ansi 2000), with emphasis on ophthalmic devices, J. Opt. Soc. Am., 24, 1250–1265.
Glickman, R. D., Jacques, S. L., Schwartz, J. A., Rodriguez, T., Lam, K.–W., and Buhr, G. (1996) Photodisruption increases the free–radical reactivity of melanosomes isolated from retinal pigment epithelium, in Laser–Tissue Interaction VII, Proc. SPIE (Jacques, S. I., ed.), Vol. 2681, SPIE, Bellingham (WA), pp. 460–467.
Van Norren, D., and Vos, J. J. (2016) Light damage to the retina: an historical approach, Eye, 30, 169–172.
Grignolo, A., Orzalesi, N., Castellazzo, R., and Vittone, P. (1969) Retinal damage by visible light in albino rats, Ophthalmologica, 157, 43–59.
Bush, R. A., Reme, C. E., and Malnoe, A. (1991) Light damage in the rat retina: the effect of dietary deprivation of n–3 fatty acids on acute structural alterations, Exp. Eye Res., 53, 741–752.
Pautler, E. L., Morita, M., and Beezley, D. (1990) Hemoprotein(s) mediate blue light damage in the retinal pigment epithelium, Photochem. Photobiol., 51, 599–605.
Demontis, G. C., Longoni, B., and Marchiafava, P. L. (2002) Molecular steps involved in light–induced oxidative damage to retinal rods, Invest. Ophthalmol. Vis. Sci., 43, 2421–2427.
Van Norren, D., and Theo, G. M. (2011) The action spectrum of photochemical damage to the retina: a review of monochromatic threshold data, Photochem. Photobiol., 87, 747–753.
Ham, W. T., Jr., Mueller, H. A., and Sliney, D. H. (1976) Retinal sensitivity to damage from short wavelength light, Nature, 260, 153–155.
Kremers, J. J., and van Norren, D. (1989) Retinal damage in macaque after white light exposures lasting ten minutes to twelve hours, Invest. Ophthalmol. Vis. Sci., 30, 1032–1040.
Sykes, S. M., Robison, W. G., Jr., Waxler, M., and Kuwabara, T. (1981) Damage to the monkey retina by broad–spectrum fluorescent light, Invest. Ophthalmol. Vis. Sci., 20, 425–434.
Ben–Shabat, S., Parish, C. A., Vollmer, H. R., Itagaki, Y., Fishkin, N., Nakanishi, K., and Sparrow, J. R. (2001) Biosynthetic studies of a2e, a major fluorophore of retinal pigment epithelial lipofuscin, J. Biol. Chem., 277, 7183–7190.
Organisciak, D. T., Wang, H. M., and Kou A. L. (1984) Ascorbate and glutathione levels in the developing normal and dystrophic rat retina: effect of intense light exposure, Curr. Eye Res., 3, 257–267.
Hunter, J. J., Morgan, J. I. W., Merigan, W. H., Sliney, D. H., Sparrow, J. R., and Williams, D. R. (2012) The susceptibility of the retina to photochemical damage from visible light, Prog. Retin. Eye Res., 31, 28–42.
Pawlak, A., Rozanowska, M., Zareba, M., Lamb, L. E., Simon, J. D., and Sarna, T. (2002) Action spectra for the photoconsumption of oxygen by human ocular lipofuscin and lipofuscin extracts, Arch. Biochem. Biophys., 403, 59–62.
Wolf, G. (2003) Lipofuscin and macular degeneration, Nutr. Rev., 61, 342–346.
Chen, Y., Sawada, O., Kohno, H., Le, Y.–Z., Subauste, C., Maeda, T., and Maeda, A. (2013) Autophagy protects the retina from light–induced degeneration, J. Biol. Chem., 288, 7506–7518.
Thumann, G., Bartz–Schmidt, K. U., Kociok, N., Kayatz, P., Heimann, K., and Schraermeyer, U. (1999) Retinal damage by light in the golden hamster: an ultrastructural study in the retinal pigment epithelium and bruch’s membrane, J. Photochem. Photobiol. B, 49, 104–111.
Hao, W., Wenzel, A., Obin, M. S., Chen, C. K., Brill, E., Krasnoperova, N. V., Eversole–Cire, P., Kleyner, Y., Taylor, A., Simon, M. I., Grimm, C., Reme, C. E., and Lem, J. (2002) Evidence for two apoptotic pathways in light–induced retinal degeneration, Nat. Genet., 32, 254–260.
Organisciak, D. T., and Vaughan, D. K. (2010) Retinal light damage: mechanisms and protection, Prog. Retin. Eye Res., 29, 113–134.
Wenzel, A., Grimm, C., Samardzija, M., and Reme, C. E. (2005) Molecular mechanisms of light–induced photore–ceptor apoptosis and neuroprotection for retinal degeneration, Prog. Retin. Eye Res., 24, 275–306.
Lieven, C. J., Ribich, J. D., Crowe, M. E., and Levin, L. A. (2012) Redox proteomic identification of visual arrestin dimerization in photoreceptor degeneration after photic injury, Invest. Ophthalmol. Vis. Sci., 53, 3990–3998.
Zernii, E. Y., Nazipova, A. A., Gancharova, O. S., Kazakov, A. S., Serebryakova, M. V., Zinchenko, D. V., Tikhomirova, N. K., Senin, I. I., Philippov, P. P., Permyakov, E. A., and Permyakov, S. E. (2015) Light–induced disulfide dimerization of recoverin under ex vivo and in vivo conditions, Free Radic. Biol. Med., 83, 283–295.
Grixti, A., Sadri, M., and Watts, M. T. (2013) Corneal protection during general anesthesia for nonocular surgery, Ocul. Surf., 11, 109–118.
Hrazdirova, V., Navratilova, B., and Ventrubova, R. (1990) Use of contact lenses during general anesthesia, Cesk. Oftalmol., 46, 223–229.
Boggild–Madsen, N. B., Bundgarrd–Nielsen, P., Hammer, U., and Jakobsen, B. (1981) Comparison of eye protection with methylcellulose and paraffin ointments during general anaesthesia, Can. Anaesth. Soc. J., 28, 575–578.
White, E., and Crosse, M. M. (1998) The aetiology and prevention of peri–operative corneal abrasions, Anaesthesia, 53, 157–161.
Cross, D. A., and Krupin, T. (1977) Implications of the effects of general anesthesia on basal tear production, Anesth. Analg., 56, 35–37.
Cuddihy, P. J., and Whittet, H. (2005) Eye observation and corneal protection during endonasal surgery, J. Laryngol. Otol., 119, 556–557.
Ganidagli, S., Cengi, M., Becerik, C., Oguz, H., and Kilic, A. (2004) Eye protection during general anaesthesia: comparison of four different methods, Eur. J. Anaesthesiol., 21, 665–667.
Manecke, G. R., Jr., Tannenbaum, D. P., and McCoy, B. E. (2000) Severe bilateral corneal injury attributed to a preservative–containing eye lubricant, Anesthesiology, 93, 1545–1546.
Zernii, E. Y., Baksheeva, V. E., Yani, E. V., Philippov, P. P., and Senin, I. I. (2017) Therapeutic proteins for treatment of corneal epithelial defects, Curr. Med. Chem., doi: 10.2174/0929867324666170609080920.
Brzheskiy, V. V., Efimova, E. L., Vorontsova, T. N., Alekseev, V. N., Gusarevich, O. G., Shaidurova, K. N., Ryabtseva, A. A., Andryukhina, O. M., Kamenskikh, T. G., Sumarokova, E. S., Miljudin, E. S., Egorov, E. A., Lebedev, O. I., Surov, A. V., Korol, A. R., Nasinnyk, I. O., Bezditko, P. A., Muzhychuk, O. P., Vygodin, V. A., Yani, E. V., Savchenko, A. Y., Karger, E. M., Fedorkin, O. N., Mironov, A. N., Ostapenko, V., Popeko, N. A., Skulachev, V. P., and Skulachev, M. V. (2015) Results of a multicenter, randomized, double–masked, placebo–controlled clinical study of the efficacy and safety of Visomitin eye drops in patients with dry eye syndrome, Adv. Ther., 32, 1263–1279.
Blades, K. J., Patel, S., and Aidoo, K. E. (2001) Oral antioxidant therapy for marginal dry eye, Eur. J. Clin. Nutr., 55, 589–597.
Xie, W. (2016) Recent advances in laser in situ keratomileusis–associated dry eye, Clin. Exp. Optom., 99, 107–112.
Kornilovskiy, I. M., Sultanova, A. I., and Burtsev, A. A. (2016) Riboflavin photoprotection with cross–linking effect in photorefractive ablation of the cornea, Vestnik Oftalmol., 132, 37–41.
McKay, T. B., and Karamichos, D. (2017) Quercetin and the ocular surface: what we know and where we are going, Exp. Biol. Med. (Maywood), 242, 565–572.
Ciuffi, M., Pisanello, M., Pagliai, G., Raimondi, L., Franchi–Micheli, S., Cantore, M., Mazzetti, L., and Failli, P. (2003) Antioxidant protection in cultured corneal cells and whole corneas submitted to UV–B exposure, J. Photochem. Photobiol. B, 71, 59–68.
Hammond, B. R., Johnson, B. A., and George, E. R. (2014) Oxidative photodegradation of ocular tissues: beneficial effects of filtering and exogenous antioxidants, Exp. Eye Res., 129, 135–150.
Gueven, N., Nadikudi, M., Daniel, A., and Chhetri, J. (2017) Targeting mitochondrial function to treat optic neuropathy, Mitochondrion, 36, 7–14.
Zueva, M. V., and Ivanina, T. A. (1980) Damaging effect of visual light on the retina in experiment (electrophysiological and electron microscopy studies), Vestnik Oftalmol., 4, 48–51.
Bhagavan, H. N., and Chopra, R. K. (2007) Plasma coenzyme q10 response to oral ingestion of coenzyme Q10 formulations, Mitochondrion, 7 (Suppl.), S78–88.
Manach, C., Williamson, G., Morand, C., Scalbert, A., and Remesy, C. (2005) Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies, Am. J. Clin. Nutr., 81, 230S–242S.
Gueven, N., Woolley, K., and Smith, J. (2015) Border between natural product and drug: comparison of the related benzoquinones idebenone and coenzyme Q10, Redox Biol., 4, 289–295.
Blagosklonny, M. V., Campisi, J., Sinclair, D. A., Bartke, A., Blasco, M. A., Bonner, W. M., Bohr, V. A., Brosh, R. M., Jr., Brunet, A., Depinho, R. A., Donehower, L. A., Finch, C. E., Finkel, T., Gorospe, M., Gudkov, A. V., Hall, M. N., Hekimi, S., Helfand, S. L., Karlseder, J., Kenyon, C., Kroemer, G., Longo, V., Nussenzweig, A., Osiewacz, H. D., Peeper, D. S., Rando, T. A., Rudolph, K. L., Sassone–Corsi, P., Serrano, M., Sharpless, N. E., Skulachev, V. P., Tilly, J. L., Tower, J., Verdin, E., and Vijg, J. (2010) Impact papers on aging in 2009, Aging (Albany NY), 2, 111–121.
Skulachev, V. P., Anisimov, V. N., Antonenko, Y. N., Bakeeva, L. E., Chernyak, B. V., Erichev, V. P., Filenko, O. F., Kalinina, N. I., Kapelko, V. I., Kolosova, N. G., Kopnin, B. P., Korshunova, G. A., Lichinitser, M. R., Obukhova, L. A., Pasyukova, E. G., Pisarenko, O. I., Roginsky, V. A., Ruuge, E. K., Senin, I. I., Severina, I. I., Skulachev, M. V., Spivak, I. M., Tashlitsky, V. N., Tkachuk, V. A., Vyssokikh, M. Y., Yaguzhinsky, L. S., and Zorov, D. B. (2009) An attempt to prevent senescence: a mitochondrial approach, Biochim. Biophys. Acta, 1787, 437–461.
Zernii, E. Y., Gancharova, O. S., Baksheeva, V. E., Golovastova, M. O., Kabanova, E. I., Savchenko, M. S., Tiulina, V. V., Sotnikova, L. F., Zamyatnin, A. A., Jr., Philippov, P. P., and Senin, I. I. (2017) Mitochondria–targeted antioxidant SkQ1 prevents anesthesia–induced dry eye syndrome, Oxid. Med. Cell. Longev., 2017, 9281519.
Vallabh, N. A., Romano, V., and Willoughby, C. E. (2017) Mitochondrial dysfunction and oxidative stress in corneal disease, Mitochondrion, 36, 103–113.
Linton, J. D., Holzhausen, L. C., Babai, N., Song, H., Miyagishima, K. J., Stearns, G. W., Lindsay, K., Wei, J., Chertov, A. O., Peters, T. A., Caffe, R., Pluk, H., Seeliger, M. W., Tanimoto, N., Fong, K., Bolton, L., Kuok, D. L., Sweet, I. R., Bartoletti, T. M., Radu, R. A., Travis, G. H., Zagotta, W. N., Townes–Anderson, E., Parker, E., Van der Zee, C. E., Sampath, A. P., Sokolov, M., Thoreson, W. B., and Hurley, J. B. (2010) Flow of energy in the outer retina in darkness and in light, Proc. Natl. Acad. Sci. USA, 107, 8599–8604.
Sacca, S. C., Roszkowska, A. M., and Izzotti, A. (2013) Environmental light and endogenous antioxidants as the main determinants of non–cancer ocular diseases, Mutat. Res., 752, 153–171.
Shimmura, S., Tadano, K., and Tsubota, K. (2004) UV dose–dependent caspase activation in a corneal epithelial cell line, Curr. Eye Res., 28, 85–92.
Sacca, S. C., Cutolo, C. A., Ferrari, D., Corazza, P., and Traverso, C. E. (2018) The eye, oxidative damage and polyunsaturated fatty acids, Nutrients, 10, E668.
Specht, S., Organisciak, D. T., Darrow, R. M., and Leffak, M. (2000) Continuing damage to rat retinal DNA during darkness following light exposure, Photochem. Photobiol., 71, 559–566.
Roginsky, V., Barsukova, T., Loshadkin, D., and Pliss, E. (2003) Substituted p–hydroquinones as inhibitors of lipid peroxidation, Chem. Phys. Lipids, 125, 49–58.
Antonenko, Y. N., Avetisyan, A. V., Bakeeva, L. E., Chernyak, B. V., Chertkov, V. A., Domnina, L. V., Ivanova, O. Y., Izyumov, D. S., Khailova, L. S., Klishin, S. S., Korshunova, G. A., Lyamzaev, K. G., Muntyan, M. S., Nepryakhina, O. K., Pashkovskaya, A. A., Pletjushkina, O. Y., Pustovidko, A. V., Roginsky, V. A., Rokitskaya, T. I., Ruuge, E. K., Saprunova, V. B., Severina, I. I., Simonyan, R. A., Skulachev, I. V., Skulachev, M. V., Sumbatyan, N. V., Sviryaeva, I. V., Tashlitsky, V. N., Vassiliev, J. M., Vyssokikh, M. Y., Yaguzhinsky, L. S., Zamyatnin, A. A., Jr., and Skulachev, V. P. (2008) Mitochondria–targeted plastoquinone derivatives as tools to interrupt execution of the aging program. 1. Cationic plastoquinone derivatives: synthesis and in vitro studies, Biochemistry (Moscow), 73, 1273–1278.
Anisimov, V. N., Bakeeva, L. E., Egormin, P. A., Filenko, O. F., Isakova, E. F., Manskikh, V. N., Mikhelson, V. M., Panteleeva, A. A., Pasyukova, E. G., Pilipenko, D. I., Piskunova, T. S., Popovich, I. G., Roshchina, N. V., Rybina, O. Y., Saprunova, V. B., Samoylova, T. A., Semenchenko, A. V., Skulachev, M. V., Spivak, I. M., Tsybul’ko, E. A., Tyndyk, M. L., Vyssokikh, M. Y., Yurova, M. N., Zabezhinsky, M. A., and Skulachev, V. P. (2008) Mitochondria–targeted plastoquinone derivatives as tools to interrupt execution of the aging program. 5. SkQ1 pro-longs lifespan and prevents development of traits of senescence, Biochemistry (Moscow), 73, 1329–1342.
Neroev, V. V., Archipova, M. M., Bakeeva, L. E., Fursova, A. Zh., Grigorian, E. N., Grishanova, A. Y., Iomdina, E. N., Ivashchenko, Zh. N., Katargina, L. A., Khoroshilova–Maslova, I. P., Kilina, O. V., Kolosova, N. G., Kopenkin, E. P., Korshunov, S. S., Kovaleva, N. A., Novikova, Y. P., Philippov, P. P., Pilipenko, D. I., Robustova, O. V., Saprunova, V. B., Senin, I. I., Skulachev, M. V., Sotnikova, L. F., Stefanova, N. A., Tikhomirova, N. K., Tsapenko, I. V., Shchipanova, A. I., Zinovkin, R. A., and Skulachev, V. P. (2008) Mitochondria–targeted plastoquinone derivatives as tools to interrupt execution of the aging program. 4. Age–related eye disease. SkQ1 returns vision to blind animals, Biochemistry (Moscow), 73, 1317–1328.
Machemer, R., and Laqua, H. (1975) Pigment epithelium proliferation in retinal detachment (massive periretinal proliferation), Am. J. Ophthalmol., 80, 1–23.
Yang, Y., Karakhanova, S., Soltek, S., Werner, J., Philippov, P. P., and Bazhin, A. V. (2012) In vivo immunoregulatory properties of the novel mitochondria–targeted antioxidant SkQ1, Mol. Immunol., 52, 19–29.
Demianenko, I. A., Vasilieva, T. V., Domnina, L. V., Dugina, V. B., Egorov, M. V., Ivanova, O. Y., Ilinskaya, O. P., Pletjushkina, O. Y., Popova, E. N., Sakharov, I. Y., Fedorov, A. V., and Chernyak, B. V. (2010) Novel mito–chondria–targeted antioxidants, “Skulachev–ion” derivatives, accelerate dermal wound healing in animals, Biochemistry (Moscow), 75, 274–280.
Demyanenko, I. A., Zakharova, V. V., Ilyinskaya, O. P., Vasilieva, T. V., Fedorov, A. V., Manskikh, V. N., Zinovkin, R. A., Pletjushkina, O. Y., Chernyak, B. V., Skulachev, V. P., and Popova, E. N. (2017) Mitochondria–targeted antioxidant SkQ1 improves dermal wound healing in genetically diabetic mice, Oxid. Med. Cell. Longev., 2017, 6408278.
Demyanenko, I. A., Popova, E. N., Zakharova, V. V., Ilyinskaya, O. P., Vasilieva, T. V., Romashchenko, V. P., Fedorov, A. V., Manskikh, V. N., Skulachev, M. V., Zinovkin, R. A., Pletjushkina, O. Y., Skulachev, V. P., and Chernyak, B. V. (2015) Mitochondria–targeted antioxidant SkQ1 improves impaired dermal wound healing in old mice, Aging (Albany NY), 7, 475–485.
Voronkova, Ya. G., Popova, T. N., Agarkov, A. A., and Zinovkin, R. A. (2015) Effect of SkQ1 on activity of the glutathione system and NADPH–generating enzymes in an experimental model of hyperglycemia, Biochemistry (Moscow), 80, 1614–1621.
Tiulina, V., Zernii, E., Baksheeva, V., Gancharova, O., Kabanova, E., Sotnikova, L., Zamyatnin, A., Philippov, P., and Senin, I. (2018) Mitochondria–targeted antioxidant SkQ1 improves corneal healing after UV–induced damage in rabbits, FEBS Open Bio, 8,215.
Novikova, Yu. P., Gancharova, O. S., Eichler, O. V., Philippov, P. P., and Grigoryan, E. N. (2014) Preventive and therapeutic effects of SkQ1–containing Visomitin eye drops against light–induced retinal degeneration, Biochemistry (Moscow), 79, 1101–1110.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Baksheeva, V.E., Gancharova, O.S., Tiulina, V.V. et al. Iatrogenic Damage of Eye Tissues: Current Problems and Possible Solutions. Biochemistry Moscow 83, 1563–1574 (2018). https://doi.org/10.1134/S0006297918120143
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297918120143