Abstract
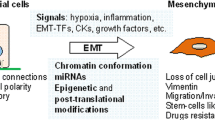
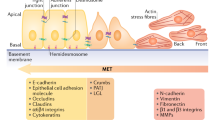
Epithelial–mesenchymal transition (EMT) is a fundamental process of morphogenesis whereby epithelial cells acquire the mesenchymal phenotype. Multiple data suggest a critical role of EMT in tumor progression. In carcinomas, EMT can be initiated and promoted by many oncogenic signaling pathways, hypoxia, and signals of tumor microenvironment resulting in epithelial cells losing their cell polarity and cell–cell adhesion and gaining the migratory and invasive properties. Downregulation of expression of the cell adhesion protein E–cadherin is considered a poor prognostic factor in cancer. Many tumors are characterized by incomplete EMT, where tumor cells acquire mesenchymal characteristics but retain their epithelial markers, in particular, E–cadherin. In cells with the hybrid epithelial–mesenchymal phenotype, E–cadherin is accumulated in adherens junctions which are less stable than adherens junctions in normal epithelial cells. E–cadherin–based adherens junctions are essential for efficient collective migration and invasion of carcinoma cells, and their survival in metastases. The plasticity of the hybrid epithelial–mesenchymal phenotype improves adaptive capabilities of cancer cells. By undergoing EMT, carcinoma cells become resistant to chemotherapy and acquire the ability to suppress immune response. Emergence of cancer stem cells after EMT activation has been observed in many types of carcinoma.
Similar content being viewed by others
Abbreviations
- EMT:
-
epithelial–mesenchymal transition
- MET:
-
mesenchymal–epithelial transition
References
Lamouille, S., Xu, J., and Derynck, R. (2014) Molecular mechanisms of epithelial–mesenchymal transition, Nat. Rev. Mol. Cell. Biol., 15, 178–196.
Nieto, M. A., Huang, R. Y., Jackson, R. A., and Thiery, J. P. (2016) EMT: 2016, Cell, 166, 21–45.
Puisieux, A., Brabletz, T., and Caramel, J. (2014) Oncogenic roles of EMT inducing transcription factors, Nat. Cell Biol., 16, 488–494.
Lambert, A. W., Pattabiraman, D. R., and Weinberg, R. A. (2017) Emerging biological principles of metastasis, Cell, 168, 670–691.
Hosseini, H., Obradovic, M. M., Hoffmann, M., Harper, K. L., Sosa, M. S., Werner–Klein, M., Nanduri, L. K., Werno, C., Ehrl, C., Maneck, M., Patwary, N., Haunschild, G., Guzvic, M., Reimelt, C., Grauvogl, M., Eichner, N., Weber, F., Hartkopf, A. D., Taran, F. A., Brucker, S. Y., Fehm, T., Rack, B., Buchholz, S., Spang, R., Meister, G., Aguirre–Ghiso, J. A., and Klein, C. A. (2016) Early dissemination seeds metastasis in breast cancer, Nature, 540, 552–558.
Linde, N., Casanova–Acebes, M., Sosa, M. S., Mortha, A., Rahman, A., Farias, E., Harper, K., Tardio, E., Reyes Torres, I., Jones, J., Condeelis, J., Merad, M., and Aguirre–Ghiso, J. A. (2018) Macrophages orchestrate breast cancer early dissemination and metastasis, Nat. Commun., 9,21.
Zhang, Y., and Weinberg, R. A. (2018) Epithelial–to–mesenchymal transition in cancer: complexity and opportunities, Front. Med., 12, 361–373.
Al–Ansari, M. M., Hendrayani, S. F., Shehata, A. I., and Aboussekhra, A. (2012) p16(INK4A) represses the paracrine tumor–promoting effects of breast stromal fibrob-lasts, Oncogene, 32, 2356–2364.
Ao, M., Franco, O. E., Park, D., Raman, D., Williams, K., and Hayward, S. W. (2007) Cross–talk between paracrine–acting cytokine and chemokine pathways promotes malignancy in benign human prostatic epithelium, Cancer Res., 67, 4244–4253.
Wendt, M. K., Smith, J. A., and Schiemann, W. P. (2010) Transforming growth factor–beta–induced epithelial–mesenchymal transition facilitates epidermal growth factor–dependent breast cancer progression, Oncogene, 29, 6485–6498.
Giannoni, E., Bianchini, F., Masieri, L., Serni, S., Torre, E., Calorini, L., and Chiarugi, P. (2010) Reciprocal activation of prostate cancer cells and cancer–associated fibrob–lasts stimulates epithelial–mesenchymal transition and cancer stemness, Cancer Res., 70, 6945–6956.
Wyckoff, J., Wang, W., Lin, E. Y., Wang, Y., Pixley, F., Stanley, E. R., Graf, T., Pollard, J. W., Segall, J., and Condeelis, J. (2004) A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors, Cancer Res., 64, 7022–7029.
Harney, A. S., Arwert, E. N., Entenberg, D., Wang, Y., Guo, P., Qian, B. Z., Oktay, M. H., Pollard, J. W., Jones, J. G., and Condeelis, J. S. (2015) Real–time imaging reveals local, transient vascular permeability, and tumor cell intravasation stimulated by TIE2hi macrophage derived VEGFA, Cancer Discov., 5, 932–943.
Berx, G., and van Roy, F. (2009) Involvement of members of the cadherin superfamily in cancer, Cold Spring Harb. Perspect. Biol., 1, a003129.
Vasioukhin, V. (2012) Adherens junctions and cancer, Subcell Biochem., 60, 379–413.
Pinheiro, H., Bordeira–Carrico, R., Seixas, S., Carvalho, J., Senz, J., Oliveira, P., Inacio, P., Gusmao, L., Rocha, J., Huntsman, D., Seruca, R., and Oliveira, C. (2010) Allele–specific CDH1 downregulation and hereditary diffuse gastric cancer, Hum. Mol. Genet., 19, 943–952.
Elloul, S., Elstrand, M. B., Nesland, J. M., Trope, C. G., Kvalheim, G., Goldberg, I., Reich, R., and Davidson, B. (2005) Snail, Slug, and Smad–interacting protein 1 as novel parameters of disease aggressiveness in metastatic ovarian and breast carcinoma, Cancer, 103, 1631–1643.
Martin, T. A., Goyal, A., Watkins, G., and Jiang, W. G. (2005) Expression of the transcription factors snail, slug, and twist and their clinical significance in human breast cancer, Ann. Surg. Oncol., 12, 488–496.
Rosivatz, E., Becker, I., Specht, K., Fricke, E., Luber, B., Busch, R., Hofler, H., and Becker, K. F. (2002) Differential expression of the epithelial–mesenchymal transition regulators Snail, SIP1, and Twist in gastric cancer, Am. J. Pathol., 161, 1881–1891.
Spaderna, S., Schmalhofer, O., Wahlbuhl, M., Dimmler, A., Bauer, K., Sultan, A., Hlubek, F., Jung, A., Strand, D., Eger, A., Kirchner, T., Behrens, J., and Brabletz, T. (2008) The transcriptional repressor ZEB1 promotes metastasis and loss of cell polarity in cancer, Cancer Res., 68, 537–544.
Tran, H. D., Luitel, K., Kim, M., Zhang, K., Longmore, G. D., and Tran, D. D. (2014) Transient SNAIL1 expression is necessary for metastatic competence in breast cancer, Cancer Res., 74, 6330–6340.
Diaz–Lopez, A., Moreno–Bueno, G., and Cano, A. (2014) Role of microRNA in epithelial to mesenchymal transition and metastasis and clinical perspectives, Cancer Manag. Res., 6, 205–216.
Liu, Y., Wang, Y., Zhang, Y., Miao, Y., Zhao, Y., Zhang, P. X., Jiang, G. Y., Zhang, J. Y., Han, Y., Lin, X. Y., Yang, L. H., Li, Q. C., Zhao, C., and Wang, E. H. (2009) Abnormal expression of p120–catenin, E–cadherin, and small GTPases is significantly associated with malignant pheno–type of human lung cancer, Lung Cancer, 63, 375–382.
Serrels, A., Canel, M., Brunton, V. G., and Frame, M. C. (2011) Src/FAK–mediated regulation of E–cadherin as a mechanism for controlling collective cell movement. Insights from in vivo imaging, Cell Adh. Migr., 5, 360–365.
Fujita, Y., Krause, G., Scheffner, M., Zechner, D., Leddy, H. E., Behrens, J., Sommer, T., and Birchmeier, W. (2002) Hakai, a c–Cbl–like protein, ubiquitinates and induces endocytosis of the E–cadherin complex, Nat. Cell Biol., 4, 222–231.
Qian, X., Karpova, T., Sheppard, A. M., McNally, J., and Lowy, D. R. (2004) E–cadherin–mediated adhesion inhibits ligand–dependent activation of diverse receptor tyrosine kinases, Embo J., 23, 1739–1748.
Perrais, M., Chen, X., Perez–Moreno, M., and Gumbiner, B. M. (2007) E–cadherin hemophilic ligation inhibits cell growth and epidermal growth factor receptor signaling independently of other cell interactions, Mol. Biol. Cell, 18, 2013–2025.
Knirsh, R., Ben–Dror, I., Spangler, B., Matthews, G. D., Kuphal, S., Bosserhoff, A. K., and Vardimon, L. (2009) Loss of E–cadherin–mediated cell–cell contacts activate a novel mechanism for up–regulation of the proto–oncogene c–Jun, Mol. Biol. Cell, 20, 2121–2129.
Zhan, T., Rindtorff, N., and Boutros, M. (2016) Wnt signaling in cancer, Oncogene, 36, 1461–1473.
Kinzler, K. W., Nilbert, M. C., Vogelstein, B., Bryan, T. M., Levy, D. B., Smith, K. J., Preisinger, A. C., Hamilton, S. R., Hedge, P., Markham, A., Carlson, M., Joslyn, G., Groden, J., White, R., Miki, Y., Miyoshi, Y., Nishisho, I., and Nakamura, Y. (1991) Identification of a gene located at chromosome 5q21 that is mutated in colorectal cancers, Science, 251, 1366–1370.
Nakamura, Y., Nishisho, I., Kinzler, K. W., Vogelstein, B., Miyoshi, Y., Miki, Y., Ando, H., Horii, A., and Nagase, H. (1991) Mutations of the adenomatous polyposis coli gene in familial polyposis coli patients and sporadic colorectal tumors, Princess Takamatsu Symp., 22, 285–292.
Korinek, V., Barker, N., Morin, P. J., van Wichen, D., de Weger, R., Kinzler, K. W., Vogelstein, B., and Clevers, H. (1997) Constitutive transcriptional activation by a beta–catenin–Tcf complex in APC–colon carcinoma, Science, 275, 1784–1787.
Wheelock, M. J., Shintani, Y., Maeda, M., Fukumoto, Y., and Johnson, K. R. (2008) Cadherin switching, J. Cell Sci., 121, 727–735.
Klymkowsky, M. W., and Savagner, P. (2009) Epithelialmesenchymal transition a cancer researcher’s conceptual friend and foe, Am. J. Pathol., 174, 1588–1593.
Jolly, M. K., Boareto, M., Huang, B., Jia, D., Lu, M., Ben–Jacob, E., Onuchic, J. N., and Levine, H. (2015) Implications of the hybrid epithelial/mesenchymal pheno–type in metastasis, Front. Oncol., 5,155.
Serrano–Gomez, S. J., Maziveyi, M., and Alahari, S. K. (2016) Regulation of epithelial–mesenchymal transition through epigenetic and post–translational modifications, Mol. Cancer, 15,18.
Hong, T., Watanabe, K., Ta, C. H., Villarreal–Ponce, A., Nie, Q., and Dai, X. (2015) An Ovol2–Zeb1 mutual inhibitory circuit governs bidirectional and multi–step transition between epithelial and mesenchymal states, PLoS Comput. Biol., 11, e1004569.
Cieply, B., Riley, P., 4th, Pifer, P. M., Widmeyer, J., Addison, J. B., Ivanov, A. V., Denvir, J., and Frisch, S. M. (2012) Suppression of the epithelial–mesenchymal transition by Grainyhead–like–2, Cancer Res., 72, 2440–2453.
Chung, V. Y., Tan, T. Z., Tan, M., Wong, M. K., Kuay, K. T., Yang, Z., Ye, J., Muller, J., Koh, C. M., Guccione, E., Thiery, J. P., and Huang, R. Y. (2016) GRHL2–miR–200–ZEB1maintains the epithelial status of ovarian cancer through transcriptional regulation and histone modification, Sci. Rep., 6, 19943.
Gregory, P. A., Bert, A. G., Paterson, E. L., Barry, S. C., Tsykin, A., Farshid, G., Vadas, M. A., Khew–Goodall, Y., and Goodall, G. J. (2008) The miR–200 family and miR–205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1, Nat. Cell Biol., 10, 593–601.
Nabeshima, K., Inoue, T., Shimao, Y., Kataoka, H., and Koono, M. (1999) Cohort migration of carcinoma cells: differentiated colorectal carcinoma cells move as coherent cell clusters or sheets, Histol. Histopathol., 14, 1183–1197.
Gillett, C. E., Miles, D. W., Ryder, K., Skilton, D., Liebman, R. D., Springall, R. J., Barnes, D. M., and Hanby, A. M. (2001) Retention of the expression of E–cadherin and catenins is associated with shorter survival in grade III ductal carcinoma of the breast, J. Pathol., 193, 33–41.
Vered, M., Allon, I., Buchner, A., and Dayan, D. (2012) Ecadherin in oral SCC: an analysis of the confusing literature and new insights related to its immunohistochemical expression, Histol. Histopathol., 27, 141–150.
Rakha, E. A., Teoh, T. K., Lee, A. H., Nolan, C. C., Ellis, I. O., and Green, A. R. (2013) Further evidence that Ecadherin is not a tumor suppressor gene in invasive ductal carcinoma of the breast: an immunohistochemical study, Histopathology, 62, 695–701.
Christiansen, J. J., and Rajasekaran, A. K. (2006) Reassessing epithelial to mesenchymal transition as a prerequisite for carcinoma invasion and metastasis, Cancer Res., 66, 8319–8326.
Cheung, K. J., Padmanaban, V., Silvestri, V., Schipper, K., Cohen, J. D., Fairchild, A. N., Gorin, M. A., Verdone, J. E., Pienta, K. J., Bader, J. S., and Ewald, A. J. (2016) Polyclonal breast cancer metastases arise from collective dissemination of keratin 14–expressing tumor cell clusters, Proc. Natl. Acad. Sci. USA, 113, E854–E863.
Yu, M., Bardia, A., Wittner, B. S., Stott, S. L., Smas, M. E., Ting, D. T., Isakoff, S. J., Ciciliano, J. C., Wells, M. N., Shah, A. M., Concannon, K. F., Donaldson, M. C., Sequist, L. V., Brachtel, E., Sgroi, D., Baselga, J., Ramaswamy, S., Toner, M., Haber, D. A., and Maheswaran, S. (2013) Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition, Science, 339, 580–584.
McInnes, L. M., Jacobson, N., Redfern, A., Dowling, A., Thompson, E. W., and Saunders, C. M. (2015) Clinical implications of circulating tumor cells of breast cancer patients: role of epithelial–mesenchymal plasticity, Front. Oncol., 5,42.
Aceto, N., Bardia, A., Miyamoto, D. T., Donaldson, M. C., Wittner, B. S., Spencer, J. A., Yu, M., Pely, A., Engstrom, A., Zhu, H., Brannigan, B. W., Kapur, R., Stott, S. L., Shioda, T., Ramaswamy, S., Ting, D. T., Lin, C. P., Toner, M., Haber, D. A., and Maheswaran, S. (2014) Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis, Cell, 158, 1110–1122.
Kowalski, P. J., Rubin, M. A., and Kleer, C. G. (2003) Ecadherin expression in primary carcinomas of the breast and its distant metastases, Breast Cancer Res., 5, R217–222.
Bukholm, I. K., Nesland, J. M., and Borresen–Dale, A. L. (2000) Re–expression of E–cadherin, α–catenin and β–catenin, but not of γ–catenin, in metastatic tissue from breast cancer patients, J. Pathol., 190, 15–19.
Ocana, O. H., Corcoles, R., Fabra, A., Moreno–Bueno, G., Acloque, H., Vega, S., Barrallo–Gimeno, A., Cano, A., and Nieto, M. A. (2012) Metastatic colonization requires the repression of the epithelial–mesenchymal transition inducer Prrx1, Cancer Cell, 22, 709–724.
Tsai, J. H., Donaher, J. L., Murphy, D. A., Chau, S., and Yang, J. (2012) Spatiotemporal regulation of epithelial–mesenchymal transition is essential for squamous cell car-cinoma metastasis, Cancer Cell, 22, 725–736.
Beerling, E., Seinstra, D., de Wit, E., Kester, L., van der Velden, D., Maynard, C., Schafer, R., van Diest, P., Voest, E., van Oudenaarden, A., Vrisekoop, N., and van Rheenen, J. (2016) Plasticity between epithelial and mesenchymal states unlinks EMT from metastasis–enhancing stem cell capacity, Cell Rep., 14, 2281–2288.
Ayollo, D. V., Zhitnyak, I. Y., Vasiliev, J. M., and Gloushankova, N. A. (2009) Rearrangements of the actin cytoskeleton and E–cadherin–based adherens junctions caused by neoplastic transformation change cell–cell interactions, PLoS One, 4, e8027.
Zhitnyak, I. Y., and Gloushankova, N. A. (2011) Morphology, cell–cell interactions, and migratory activity of IAR–2 epithelial cells transformed with the RAS onco-gene: contribution of cell adhesion protein E–cadherin, Rus. J. Develop. Biol., 42, 402–411.
Rubtsova, S. N., Zhitnyak, I. Y., and Gloushankova, N. A. (2015) A novel role of E–cadherin–based adherens junctions in neoplastic cell dissemination, PLoS One, 10, e0133578.
Meng, W., Mushika, Y., Ichii, T., and Takeichi, M. (2008) Anchorage of microtubule minus ends to adherens junctions regulates epithelial cell–cell contacts, Cell, 135, 948–959.
Pulimeno, P., Bauer, C., Stutz, J., and Citi, S. (2010) PLEKHA7 is an adherens junction protein with a tissue distribution and subcellular localization distinct from ZO–1 and E–cadherin, PLoS One, 5, e12207.
Kourtidis, A., Ngok, S. P., Pulimeno, P., Feathers, R. W., Carpio, L. R., Baker, T. R., Carr, J. M., Yan, I. K., Borges, S., Perez, E. A., Storz, P., Copland, J. A., Patel, T., Thompson, E. A., Citi, S., and Anastasiadis, P. Z. (2015) Distinct E–cadherin–based complexes regulate cell behavior through miRNA processing or Src and p120 catenin activity, Nat. Cell Biol., 17, 1145–1157.
Kourtidis, A., Necela, B., Lin, W. H., Lu, R., Feathers, R. W., Asmann, Y. W., Thompson, E. A., and Anastasiadis, P. Z. (2017) Cadherin complexes recruit mRNAs and RISC to regulate epithelial cell signaling, J. Cell Biol., 216, 3073–3085.
Kourtidis, A., Lu, R., Pence, L. J., and Anastasiadis, P. Z. (2017) A central role for cadherin signaling in cancer, Exp. Cell Res., 358, 78–85.
Mani, S. A., Guo, W., Liao, M. J., Eaton, E. N., Ayyanan, A., Zhou, A. Y., Brooks, M., Reinhard, F., Zhang, C. C., Shipitsin, M., Campbell, L. L., Polyak, K., Brisken, C., Yang, J., and Weinberg, R. A. (2008) The epithelial–mesenchymal transition generates cells with properties of stem cells, Cell, 133, 704–715.
Rasheed, Z. A., Yang, J., Wang, Q., Kowalski, J., Freed, I., Murter, C., Hong, S. M., Koorstra, J. B., Rajeshkumar, N. V., He, X., Goggins, M., Iacobuzio–Donahue, C., Berman, D. M., Laheru, D., Jimeno, A., Hidalgo, M., Maitra, A., and Matsui, W. (2010) Prognostic significance of tumorigenic cells with mesenchymal features in pancreatic adenocarcinoma, J. Natl. Cancer Inst., 102, 340–351.
Kong, D., Banerjee, S., Ahmad, A., Li, Y., Wang, Z., Sethi, S., and Sarkar, F. H. (2010) Epithelial to mesenchymal transition is mechanistically linked with stem cell signatures in prostate cancer cells, PLoS One, 5, e12445.
Fan, F., Samuel, S., Evans, K. W., Lu, J., Xia, L., Zhou, Y., Sceusi, E., Tozzi, F., Ye, X. C., Mani, S. A., and Ellis, L. M. (2012) Overexpression of snail induces epithelial–mesenchymal transition and a cancer stem cell–like phenotype in human colorectal cancer cells, Cancer Med., 1, 5–16.
Long, H., Xiang, T., Qi, W., Huang, J., Chen, J., He, L., Liang, Z., Guo, B., Li, Y., Xie, R., and Zhu, B. (2015) CD133+ ovarian cancer stem–like cells promote non–stem cancer cell metastasis via CCL5 induced epithelial–mesenchymal transition, Oncotarget, 6, 5846–5859.
Chaffer, C. L., Brueckmann, I., Scheel, C., Kaestli, A. J., Wiggins, P. A., Rodrigues, L. O., Brooks, M., Reinhardt, F., Su, Y., Polyak, K., Arendt, L. M., Kuperwasser, C., Bierie, B., and Weinberg, R. A. (2011) Normal and neoplastic non–stem cells can spontaneously convert to a stem–like state, Proc. Natl. Acad. Sci. USA, 108, 7950–7955.
Yang, A. D., Fan, F., Camp, E. R., van Buren, G., Liu, W., Somcio, R., Gray, M. J., Cheng, H., Hoff, P. M., and Ellis, L. M. (2006) Chronic oxaliplatin resistance induces epithelial–to–mesenchymal transition in colorectal cancer cell lines, Clin. Cancer Res., 12, 4147–4153.
Kajiyama, H., Shibata, K., Terauchi, M., Yamashita, M., Ino, K., Nawa, A., and Kikkawa, F. (2007) Chemoresistance to paclitaxel induces epithelial–mesenchymal transition and enhances metastatic potential for epithelial ovarian carcinoma cells, Int. J. Oncol., 31, 277–283.
Li, Q. Q., Xu, J. D., Wang, W. J., Cao, X. X., Chen, Q., Tang, F., Chen, Z. Q., Liu, X. P., and Xu, Z. D. (2009) Twist1–mediated adriamycin–induced epithelial–mesenchymal transition relates to multidrug resistance and invasive potential in breast cancer cells, Clin. Cancer Res., 15, 2657–2665.
Kajita, M., McClinic, K. N., and Wade, P. A. (2004) Aberrant expression of the transcription factors snail and slug alters the response to genotoxic stress, Mol. Cell Biol., 24, 7559–7566.
Kurrey, N. K., Jalgaonkar, S. P., Joglekar, A. V., Ghanate, A. D., Chaskar, P. D., Doiphode, R. Y., and Bapat, S. A. (2009) Snail and slug mediate radioresistance and chemoresistance by antagonizing p53–mediated apoptosis and acquiring a stem–like phenotype in ovarian cancer cells, Stem Cells, 27, 2059–2068.
Vega, S., Morales, A. V., Ocana, O. H., Valdes, F., Fabregat, I., and Nieto, M. A. (2004) Snail blocks the cell cycle and confers resistance to cell death, Genes Dev., 18, 1131–1143.
Saxena, M., Stephens, M. A., Pathak, H., and Rangarajan, A. (2011) Transcription factors that mediate epithelial–mesenchymal transition lead to multidrug resistance by upregulating ABC transporters, Cell Death Dis., 2, e179–e179.
Kudo–Saito, C., Shirako, H., Ohike, M., Tsukamoto, N., and Kawakami, Y. (2013) CCL2 is critical for immunosup-pression to promote cancer metastasis, Clin. Exp. Metastasis, 30, 393–405.
Akalay, I., Janji, B., Hasmim, M., Noman, M. Z., Andre, F., De Cremoux, P., Bertheau, P., Badoual, C., Vielh, P., Larsen, A. K., Sabbah, M., Tan, T. Z., Keira, J. H., Hung, N. T., Thiery, J. P., Mami–Chouaib, F., and Chouaib, S. (2013) Epithelial–to–mesenchymal transition and autophagy induction in breast carcinoma promote escape from T–cell–mediated lysis, Cancer Res., 73, 2418–2427.
Chen, L., Gibbons, D. L., Goswami, S., Cortez, M. A., Ahn, Y. H., Byers, L. A., Zhang, X., Yi, X., Dwyer, D., Lin, W., Diao, L., Wang, J., Roybal, J., Patel, M., Ungewiss, C., Peng, D., Antonia, S., Mediavilla–Varela, M., Robertson, G., Suraokar, M., Welsh, J. W., Erez, B., Wistuba, I. I., Chen, L., Peng, D., Wang, S., Ullrich, S. E., Heymach, J. V., Kurie, J. M., and Qin, F. X. F. (2014) Metastasis is regulated via microRNA–200/ZEB1 axis control of tumor cell PD–L1 expression and intratumoral immunosuppression, Nat. Commun., 5, 5241.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © N. A. Gloushankova, I. Y. Zhitnyak, S. N. Rubtsova, 2018, published in Biokhimiya, 2018, Vol. 83, No. 12, pp. 1802–1811.
Rights and permissions
About this article
Cite this article
Gloushankova, N.A., Zhitnyak, I.Y. & Rubtsova, S.N. Role of Epithelial-Mesenchymal Transition in Tumor Progression. Biochemistry Moscow 83, 1469–1476 (2018). https://doi.org/10.1134/S0006297918120052
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297918120052