Abstract
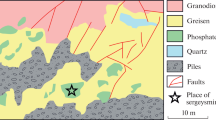
Karchevskyite, a new mineral related to the family of layered double hydroxides (LDHs), has been found in the Iron open pit at the Kovdor carbonatite massif, Kola Peninsula, Russia. The mineral occurs as spherulites of up to 1.5 mm in diameter composed of thin, curved lamellae. Dolomite, magnetite, quintinite-3T, strontium carbonate, and fluorapatite are associated minerals. Karchevskyite is white in aggregates and colorless in separate platelets. Its luster is vitreous with a pearly shine on the cleavage surface. The new mineral is nonfluorescent. The Mohs hardness is 2. The cleavage is eminent (micalike), parallel to {001}. The measured density is 2.21(2) g/cm3, and the calculated value is 2.18(1) g/cm3. Karchevskyite is colorless and nonpleochroic in immersion liquids. It is uniaxial, negative, ω = 1.542(2), and ɛ = 1.534(2). The chemical composition (electron microprobe, average of ten point analyses, standard deviation in parentheses, wt %) is as follows: 29.7(1.1) MgO, 18.3(0.7) Al2O3, 7.4(0.4) SrO, 0.2(0.1) CaO, 1.3(0.2) P2O5, 14.5(0.4) CO2, and 28.6 H2O (estimated by difference); the total is 100. The empirical formula calculated on the basis of nine Al atoms is Mg18.00Al9.00(OH)54.00(Sr1.79Mg0.48Ca0.09)2.36 (Ca3)8.26(PO4)0.46(H2O)6.54(H3O)4.18. The idealized formula is [Mg18Al9(OH)54][Sr2(CO3, PO4)9(H2O, H3O)11]. The new mineral slowly dissolves in 10% HCl with weak effervescence. Karchevskyite is trigonal; possible space groups are P3, P3, P \( \overline 3 \) 1m, P31m, P312, P312, P3m1, or P3m1; unit-cell dimensions are a = 16.055(6), c = 25.66(1) Å, V = 5728(7) Å3, Z = 3. The strongest reflections in the X-ray powder diffraction pattern [d, (I, %)(hkl)] are: 8.52(10)(003), 6.41(4)(004), 5.13(3)(005), 4.27(6)(006), 3.665(9)(007), 3.547(9)(107), 3.081(6)(315). Wavenumbers of absorption bands in the infrared spectrum of the new mineral are (cm−1; s is shoulder): 3470, 3420s, 3035, 2960s, 1650, 1426, 1366, 1024, 937, 860, 779, 678, 615s, 553, 449, 386. Results of thermogravimetric analysis: total weight loss is 42.0 wt %, with three stages of loss: 12.2%, maximum rate at 230°C; 6.1%, maximum rate at 320°C; and 23.7%, maximum rate at 440°C. Karchevskyite is a late-stage hydrothermal mineral. The mineral is named in memory of Russian mineralogist Pavel Karchevsky (1976–2002), who made a significant contribution to the study of carbonatites. The type material of karchevskyite is deposited at the Mineralogical Museum, Division of Mineralogy, St. Petersburg State University, and the Fersman Mineralogical Museum, Russian Academy of Sciences, Moscow.
Similar content being viewed by others
References
M. Bellotto, B. Rebours, O. Clause, et al., “A Reexamination of Hydrotalcite Chemistry,” J. Phys. Chem. 100, 8527–8534 (1996).
S. N. Britvin, Ya. A. Pakhomovskii, A. N. Bogdanova, and V. I. Skba, “Strontiowhitlockite, Sr9Mg(PO3OH)(PO4)6, a New Mineral Species from the Kovdor Deposit, Kola Peninsula, USSR,” Can. Mineral. 29, 87–93 (1991).
S. N. Britvin, G. Ferraris, G. Ivaldi, et al., “Cattite, Mg3(PO4)2 · 22H2O, a New Mineral from Zhelezny Mine (Kovdor Massif, Kola Peninsula, Russia),” N. Jb. Miner. Monatshefte, No. 4, 160–168 (2002).
A. G. Bulakh and V. V. Ivanikov, Mineralogy and Petrology of Carbonatites (Leningrad State Univ., Leningrad, 1982) [in Russian].
G. Chao and R. A. Gaylt, “Quintinite-2H, Quintinite-3T, Charmarite-2H, Charmarite-3T and Caresite-3T, a New Group of Carbonate Minerals Related to the Hydrotalcite-Manasseite Group,” Can. Mineral. 35, 1541–1549 (1997).
M. J. Hermamdez-Moreno, M. A. Ulbarri, J. L. Rendon, and C. J. Serna, “IR Characteristics of Hydrotalcite-Like Compounds,” Phys. Chem. Miner. 12, 34–38 (1985).
P. I. Karchevsky, Sulfide, Strontium, and Rare Earth Mineralization of Phoscorite and Carbonatite in the Tur’in Massiv (Russia) and the Lyulekop Deposits (South Africa Republic) (Dom Kolo, St. Petersburg, 2005) [in Russian].
P. I. Karchevsky and J. Moutte, “The Phoscorite-Carbonatite Complex of Vuoriyarvi, Northern Karelia,” in Phoscorites and Carbonatites from Mantle to Mine: The Key Example of the Kola Alkaline Province, Ed. by F. Wall and A. N. Zaitsev (Mineral. Soc. Great Britain, London, 2004), pp. 163–200.
A. I. Khan and D. O’Hare, “Intercalation Chemistry of Layered Double Hydroxides: Recent Developments and Applications,” J. Mater. Chem. 12, 3191–3198 (2002).
J. T. Kloprogge, D. Wharton, L. Hickey, and R. L. Frost, “Infrared and Raman Study of Interlayer Anions CO 2−3 , NO −3 , SO −4 and ClO −4 in Mg/Al Hydrotalcite,” Am. Mineral. 87, 623–629 (2002).
N. I. Krasnova, “The Kovdor Phlogopite Deposit, Kola Peninsula, Russia,” Can. Mineral. 39, 33–44 (2001).
A. A. Kukharenko, M. P. Orlova, A. G. Bulakh, et al., Caledonian Complex of Ultramafic Alkaline Rocks and Carbonatite of the Kola Peninsula and Northern Karelia. Geology, Petrology, Mineralogy, Geochemistry (Nedra, Moscow, 1965) [in Russian].
J. A. Mandarino, “The Gladstone-Dale Relationship. III. Some General Applications,” Can. Mineral. 17, 71–76 (1979).
S. Miyata, “The Synthesis of Hydrotalcite-Like Compounds and Their Structures and Physical-Chemical Properties: I. the Systems Mg2+-Al3+-NO −3 , Mg2+-Al3+-ClO−, Mg2+-Al3+-ClO −4 , Ni2+-Al3+-Cl−, and Zn2+-Al3+-Cl−,” Clays Clay Mineral. 23, 369–375 (1975).
S. Miyata and T. Kimura, “Synthesis of New Hydrotalcite-Like Compounds and Their Physical-Chemical Properties,” Chem. Lett., 843–848 (1973).
S. Miyata and A. Okada, “Synthesis of Hydrotalcite-Like Compounds and Their Physico-Chemical Properties — the System Mg2+-Al3+-SO 2−4 and Mg2+-Al3+-CrO 2−4 ,” Clays Clay Miner. 25, 14–18 (1977).
P. B. Moore, “Wermlandite, a New Mineral from Langban, Sweden,” Lithos 4, 213–217 (1971).
G. Moreau, L. Heln, J. Purans, and A. E. Merbach, “Structural Investigation of Aqueous Eu2+ Ion: Comparison with Sr2+ Using the XAFS Technique,” J. Phys. Chem. A106, 3034–3043 (2002).
F. Prinetto, G. Ghiotti, P. Graffin, and D. Tichit, “Synthesis and Characterization of Sol-Gel Mg/Al and Ni/Al Layered Double Hydroxides and Comparison with Co-Precipitated Samples,” Microporous and Mesoporous Mater. 39, 229–247 (2000).
F. Rey, V. Fornes, and J. M. Rojo, “Thermal Decomposition of Hydrotalcites. An Infrared and Nuclear Magnetic Resonance Spectroscopic Study,” J. Chem. Soc. Faraday Trans. 88, 2233–2238 (1992).
J. S. Ricci, R. C. Stevens, R. K. McMullan, and W. T. Klooster, “Structure of Strontium Hydroxide Octahydrate, Sr(OH)2 · 8H2O, at 20, 100 and 200 K from Neutron Diffraction,” Acta Cryst. B61, 381–386 (2005).
O. M. Rimskaya-Korsakova and N. I. Krasnova, Geology of Mineral Deposits in the Kovdor Massif (St. Petersburg State Univ., St. Petersburg, 2002) [in Russian].
J. Rius and R. Allmann, “Structure of Wermlandites, [Mg7(Al, Fe)2(OH)18]2+[Ca(H2O)62SO46H2O]2−,” Fortschritt. Miner. 56, 113–114 (1978).
J. Rius and R. Allmann, “The Superstructure of the Double Layer Mineral Wermlandite [Mg7(Al0.57, Fe0.433)(OH)18]2+ [(Ca0.6, Mg0.4)(SO4)2(H2O)12]2−,” Z. Kristallogr. 168, 133–144 (1984).
J. Rius and F. Plana, “Contribution to the Superstructure Resolution of the Double Layer Mineral Motukoreaite,” N. Jb. Miner. Monatshefte, No. 6, 263–272 (1986).
K. A. Rodgers, J. E. Chisholm, R. J. Davis, and C. S. Nelson, “Motukoreaite, a New Hydrated Carbonate, Sulfate, and Hydroxide of Magnesium and Aluminum from Auckland, New Zealand,” Mineral. Mag. 41, 389–390 (1977).
J. C. A. A. Roelofs, J. A. van Bokhoven, A. Jos Van Dillen, et al., “The Thermal Decomposition of Mg/Al Hydrotalcites: Effects of Interlayer Anions and Characteristics of the Final Structure,” Chem. Eur. J. 8, 5571–5579 (2000).
V. S. Samoilov, Carbonatites. Facies and Formation Conditions (Nauka, Moscow, 1977) [in Russian].
H. G. Smith, “The Crystal Structure of Strontium Hydroxide Octahydrate, Sr(OH)2(H2O)8,” Acta Crystallogr. 6, 604–609 (1953).
M. Ya. Somina, Dolomite and Ankerite Carbonatites in East Siberia (Nedra, Moscow, 1975) [in Russian].
Ts. Stanimirova, N. Piperov, N. Petrova, and G. Kirov, “Thermal Evolution of Mg-Al-CO3 Hydrotalcites,” Clay Mineral. 39, 177–191 (2004).
H. Strunz and E. H. Nickel, Strunz Mineralogical Tables (Schweizerbart, Stuttgart, 2001).
Phosphorites and Carbonatites from Mantle to Mine: The Key Example of the Kola Alkaline Province, Ed. by F. Wall and A. N. Zaitsev (Mineral. Soc. Great Britain, London, 2004).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © S.N. Britvin, N.V. Chukanov, G.K. Bekenova, M.A. Yagovkina, A.V. Antonov, A.N. Bogdanova, N.I. Krasnova, 2007, published in Zapiski Rossiiskogo Mineralogicheskogo Obshchestva, 2007, No. 5, pp. 44–56.
The new mineral karchevskyite and its name accepted by the Commission on New Minerals and Mineral Names, Russian Mineralogical Society, March 21, 2005. Approved by the Commission on New Minerals and Mineral Names, International Mineralogical Association, June 30, 2005.
Rights and permissions
About this article
Cite this article
Britvin, S.N., Chukanov, N.V., Bekenova, G.K. et al. Karchevskyite, [Mg18Al9(OH)54][Sr2(CO3,PO4)9(H2O,H3O)11], a new mineral species of the layered double hydroxide family. Geol. Ore Deposits 50, 556–564 (2008). https://doi.org/10.1134/S1075701508070064
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1075701508070064