Abstract
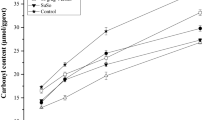
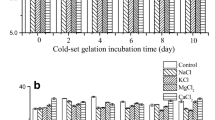
The cryoprotective effects of trehalose on fish myofibrillar protein were compared with those of sucrose, glucose and sorbitol. The frozen surimi with trehalose exhibited significantly higher Ca2+-ATPase activity through-out the storage periods, resulting in higher gel-forming ability than that of without trehalose. The amount of unfrozen water was significantly increased in the surimi upon addition of trehalose at any concentrations tested. The findings suggest that trehalose constructed bound water molecules in protein structure, consequently suppressed freeze-induced denaturation of protein and maintained gel-forming ability. An addition of 5.0% to 7.5% concentration of trehalose showed threshold behavior to increase the amount of unfrozen water and to prevent freeze-induced denaturation of protein. The effects of trehalose were almost similar to those of other sugars.
Similar content being viewed by others
References
Dyer WJ. Protein denaturation in frozen and stored fish. Food Res. 1951; 16: 522–527.
Noguchi S, Matsumoto JJ. Studies on the control of the denaturation of the fish muscle proteins during frozen storage-I. Preventive effect of Na-glutamate. Nippon Suisan Gakkaishi 1970; 36: 1078–1087.
Matsumoto JJ. Protein at low temperature. In: Fennema O (ed). Journal of American Chemical Society; Advances in Chemistry Series 180. Association of Official Analytical Chemists, Arlington, VA, 1979; 205–224
Hossain MA, Khan MAA, Osako K, Ishihara T, Hara K, Osatomi K, Nozaki Y. Effect of proteolytic squid protein hydrolysate on textural quality and denaturation of wanieso lizardfish (Saurida wanieso) surimi during frozen storage. Trans. JSRAE 2003; 20: 317–324.
Matsumoto I, Ooizumi T, Arai K. Protective effect of sugar on freeze-denaturation of carp myofibrillar protein. Nippon Suisan Gakkaishi 1985; 51: 833–839.
Park JW. Functional protein additives in surimi gel. J. Food Sci. 1994; 59: 525–527.
Park JW, Lanier TC. Combined effects of phosphates and sugar or polyol on protein stabilization of fish myofibrils. J. Food Sci. 1987; 52: 1509–1513.
Hanafusa N. High polymer of organism and water. In: Nippon Suisan Gakkai (ed). Water in Foods. Kouseisha Kouseikaku, Tokyo, 1973; 9–24.
Kavanau JL. Water. In: Kavanau JL (ed). Structure and Function in Biological Membrane. Holden Day, San Francisco, CA. 1965; 171–248.
Nemethy G, Scheraga HA. Structure of water and hydrophobic bonding in protein-I. A model for the thermodynamic properties of liquid water. J. Chem. Phys. 1962; 36: 3382–3400.
Nemethy G, Scheraga HA. Structure of water and hydrophobic bonding in protein-I. A model for the thermodynamic properties of aqueous solution of hydrocarbon. J. Chem. Phys. 1962; 36: 3401–3417.
Oku K, Sawatani I, Chaen H, Fukuda S, Kurimoto M. Trehalose content in foods. Nippon Shokuhin Kagaku Kogaku Kaishi 1998; 45: 381–384.
Stewart LC, Richtmyer NK, Hudson CS. The preparation of trehalose from yeast. J. Am. Chem. Soc. 1950; 72: 2059–2061.
Tabuchi A, Mandai T, Shibuya T, Fukuda S, Sugimoto T, Kurimoto M. Formation of trehalose from starch by novel enzyme. Oyo Toshitsu Kagaku 1995; 42: 401–406.
Sakurai M, Inoue Y. Hydration characteristics of carbohydrates and physiological functions of trehalose. Biophysics 1997; 37: 326–330.
Williams S. Official Methods of Analysis of the Association of Official Analytical Chemists, 14th edn. Association of Official Analytical Chemists, Arlington, VA. 1984; 152–159.
Niwa E, Nowsad AA, Kanoh S. Comparative studies on the physical parameters of Kamaboko treated with low temperature setting and high temperature setting. Nippon Suisan Gakkaishi 1991; 57: 105–109.
Lanier TC, Hart K, Martin RE (eds). A Manual of Standard Methods for Measuring and Specifying the Properties of Surimi. National Fisheries Institute, Washington, DC. 1991; 61.
Katoh N, Uchiyama H, Tsukamoto S, Arai K. A biochemical study on fish myofibrillar ATPase. Nippon Suisan Gakkaishi 1977; 43: 857–867.
Gornall AG, Bardawill CT, David MM. Determination of serum proteins by means of the Biuret reaction. J. Biol. Chem. 1949; 177; 751–766.
Wakamatsu T, Sato Y. Determination of unfreezable water in sucrose, sodium chloride and protein solutions by differential scanning calorimater. Nippon Nogeikagaku Kaishi 1979; 53: 415–420.
Okada T, Inoue N, Shinano H. An explanation of freeze denaturation on the kinetics of thermal inactivation of frozen-stored carp myosin B. Nippon Suisan Gakkaishi 1986; 52: 1765–1770.
Inoue N, Takatori K, Motoshige T. Effect of storage temperature on the freeze denaturation of fish myosin B. Nippon Suisan Gakkaishi 1992; 58: 2357–2360.
Kitazawa H, Kawai Y, Inoue N, Shinano H. Influence on the decrease of Ca2+-ATPase activity and solubility of carp myofibrils during frozen storage. Fish. Sci. 1995; 61: 1037–1038.
Yoshikawa K, Inoue N, Kawai Y, Shinano H. Changes of the solubility and ATPase activity of carp myofibrils during frozen storage at different temperatures. Fish. Sci. 1995; 61: 804–812.
Yoshikawa K, Inoue N, Kawai Y, Shinano H. Subunit components in salt-soluble and insoluble fractions of carp myofibrils during frozen storage. Fish. Sci. 1995; 61: 813–816.
Kitazawa H, Kawai Y, Inoue N, Shinano H. Influence of KCl on the decrease of Ca2+-ATPase activity and solubility of carp myofibrils during frozen storage. Fish. Sci. 1995; 61: 1037–1038.
Ohnishi M, Tsuchiya T, Matsumoto J. Kinetic study on the denaturation mechanism of carp actomyosin during frozen storage. Nippon Suisan Gakkaishi 1978; 44: 27–37.
Dyer WJ, Fraser DI. Protein in fish muscle-13. Lipid hydrolysis. J. Fish. Res. Bd. Can. 1959; 16: 43–52.
Finn DB. The denaturation of fish muscle proteins by freezing. Contr. Can. Biol. Fish. N. S. 1934; 8: 311–320.
Sych J, Lacroix C, Adambounou T, Castaigne F, Cryoprotective effects of some materials on cod-surimi proteins during frozen storage. J. Food Sci. 1990; 55: 1222–1227.
Ramirez JA, Martin-Polo MO, Bandman E. Fish myosin aggregation, as affected by freezing and initial physical state. J. Food Sci. 2000; 65: 556–560.
Samejima K, Oka Y, Yamamoto K. Studies on heated-induced gelation of cardiac myosin and actomyosin. Part II. Effects of SH groups, ε-NH2 groups, ATP, and myosin subfragments on heat-induced gelling of cardiac myosin and comparison with skeletal myosin and actomyosin gelling capacity. Agric. Biol. Chem. 1988; 52: 63–70.
Kaminishi Y, Miki H, Isohata T, Nishimoto J. Effects of temperature on reactions of heat-induced gel formation in smooth dogfish muscle. Nippon Suisan Gakkaishi 1990; 56: 1285–1292.
Azuma Y, Konno K. Freeze denaturation of carp myofibrils compared with thermal denaturation. Fish. Sci. 1998; 64: 287–290.
Funatsu Y, Hosokawa H, Nanbu S, Arai K. Effects of sorbitol on gelation and cross-linking of myosin heavy chain of salt ground meat from walleye pollack. Nippon Suisan Gakkaishi 1993; 59: 1599–1607.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Osako, K., Hossain, M.A., Kuwahara, K. et al. Effect of trehalose on the gel-forming ability, state of water and myofibril denaturation of horse mackerel Trachurus japonicus surimi during frozen storage. Fish Sci 71, 367–373 (2005). https://doi.org/10.1111/j.1444-2906.2005.00973.x
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1111/j.1444-2906.2005.00973.x