Summary
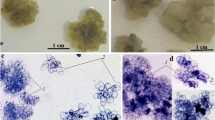
Callus induction and regeneration studies were carried out on a medicinal fern, Drynaria quercifolia native to Asian countries. It is a seasonal fern that regenerates only during the monsoons. Callus was induced on Knop’s (1865) medium supplemented with 20 gl−1 sucrose, 8gl−1 agar, and either 2,4-dichlorophenoxyacetic acid (2,4-D), 2,4,5-trichlorophenoxyacetic acid (2,4,5-T), 4-amino-3,5,6-trichloropicolinic acid (picloram), or indole-3-butyric acid at different concentrations. Morphogenetic callus obtained on 5 mgl−1 2,4,5-T was subcultured onto solid and liquid media (shaken flask and discontinuously stirred bioreactor cultures) for callus proliferation and regeneration studies. A significant amount of sporophyte regeneration was observed on solid medium containing 10 mgl−1 6-(δ, δ-dimethylallylamino) purine (2iP). Sporophyte regeneration from callus followed an atypical pattern of development. Leafy structures of single-cell thickness with a microrhizome were formed as sporophyte initials. Prolonged cultures of these structures resulted in the formation of juvenile sporophytes in vitro. The use of liquid media resulted in increased biomass in culture. The present study is the first report of a successful system for callus production and regeneration of sporophytes from leafy structures in ferns. The method can be successfully applied for generation of biomass of D. quercifolia, throughout the year.
Similar content being viewed by others
References
Ambasta, S. P. The useful plant of India. New Delhi: CSIR Publications and Information Directorate; 1992:918 pp.
Bristow, J. M. The controlled in vitro differentiation of callus derived from fern Pteris cretica L. into gametophytic or sporophytic tissues. Dev. Biol. 4:361–375; 1962.
Bryne, T. E.; Caponetti, J. Morphogenesis in three cultivars of Boston fern II. Callus production from stolon tips and plantlet differentiation from callus. Am. Fern. J. 82(1):1–11; 1992.
Chatterjee, A.; Prakashi, S. C., The treatise on Indian medicinal plants. vol:1. New Delhi: CSIR Publications and Information Directorate; 1991:172 pp.
Cheema, H. K.; Kaur, M. In vitro regeneration and effect of growth regulators on aquatic heterosporous ferns. Res. Bull. Chd. (Science) 36:35–37; 1985.
Cheema, H. K.; Sharma, M. B. Induction of multiple shoots from adventitious buds and leaf callus in Ceratopteris thalictroides. Indian Fern J. 11:63–67; 1994.
Chopra, R. N.; Nayar, S. L.; Chopra, I. C. Glossary of Indian Medicinal Plants. CSIR New Delhi: Publications and information directorate; 1992;330 pp.
Constable, F.; Tyler, R. T. Cell culture for production of secondary metabolites. In: Vasil, I. K.; Thorpe, T. A., eds. Plant cell and tissue culture. Dordrecht: Kluwer Academic Publishers; 1994:271–289.
Debergh, P. In vitro culture of ornamentals. In: Vasil, I. K.; Thorpe, T. A., eds. Plant cell and tissue culture. Dordrecht: Kluwer Academic Publishers; 1994;561–573.
Dolinsek, A.; Camloh, M. Gametophytic and Sporophytic regeneration from bud scales of the fern Platycerium bifurcatum (Cav.) C. Chr.in vitro. Ann. Bot. 80:23–28; 1997.
Fernandez, H.; Bertrand, A. M.; Sanchez-Tames, R., Influence of tissue culture conditions on apogamy in Dryopteris affinis sp. Affinis. Plant Cell Tiss. Organ Cult. 45:93–97; 1996.
Fernandez, H.; Revilla, M. A. In vitro culture of ornamental ferns. Plant Cell. Tiss. Organ Cult. 73:1–13; 2003.
George, E. F., ed. Plant Propagation and Micropropagation. Plant propagation by tissue culture. Part I The Technology. Wiltshire, England: Exegetics Ltd; 1993:574 pp.
Hegde, S.; D’Souza, L. Effect of season on contamination and in vitro germination of the spores of Drynaria quercifolia. Indian Fern J. 16:30–34; 1999.
Hegde, S.; D’Souza, L. In vitro sporophyte production from rhizome of Drynaria quercifolia. Indian Fern J. 17:72–76; 2000.
Hegde, S.; D’Souza, L. Axenic culture of Drynaria quercifolia (L) from spores for ornamental purposes. In: Kumar, N.; Negi, P. S.; Singh, N. K., eds. Plant biotechnology for sustainable hill agriculture. Pithoragarh, India: Defence Agricultural Research Laboratory; 2002a:74–77.
Hegde, S.; D’Souza, L. 2iP: An effective cytokinin for the tissue culture of ferns. The Fern Gaz. 6:45; 2002b.
Hegde, S.; D’Souza, L. Influence of nutrition on in vitro germination and development of gametophytes of Drynaria quercifolia. Indian Fern J. 20:71–77; 2003.
Kato, Y. Physiological and Morphogenetic studies of fern gametophytes in aseptic cultures I. Callus tissue from dark cultured Pteris vittata. Bot. Gaz. 124:413–416; 1963.
Knop, W. Quantitative Utersuchungen Über den Ernährungensprozeß der Pflanze. Landw. Versuchssat. 7:93; 1865.
Laetsch, W. M.; Briggs, W. R. Kinetin modification of sporeling ontogeny in Marsilea vestiata. Am. J. Bot. 48:369–377; 1961.
Mehra, P. N.; Palta, H. K. In vitro controlled differentiation of the root callus of Cyclosorus dentatus. Phytomorphology 21:367–375; 1971.
Mehra, P. N.; Sulklyan, D. S. In vitro studies on apogamy, apospory and controlled differentiation of rhizome segments of the fern Ampelopteris prolifera (Retz) Copel. Bot. J. Linn. Soc. 62:431–433; 1969.
Murashige, T. Current status of plant cell and organ culture. HortScience 12:3–6; 1974.
Murashige, T.; Skoog, T. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant 15:473–497; 1962.
Partanen, C. R. Comparison of gametophytic callus and tumours of Pteridium aquilinum. Bot. Gaz. 133:287–292; 1972.
Peterson, R. L. Callus induction in Ophioglossum petiolatum Hook. Can. J. Bot. 45:2225–2227; 1967.
Phillips, G. C. In vitro morphogenesis in plants. In Vitro Cell. Dev. Biol. Plant 40:342–345; 2004.
Singh, F. In vitro Orchid seed germination and cloning of orchids—A success story. In: Prakash, J.; Pierik, R. L. M., eds. Plant biotechnology Commercial prospects and Problems. New Delhi, Bombay, Calcutta: Oxford & IBH Publishing Co. Pvt. Ltd.; 1993:85–109.
Vashishta, P. C., ed. Botany. Part IV. Pteridophyta (Vascular Cryptogams), 5th ed, New Delhi Chand S and Company Ltd.; 1997:390 pp.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hegde, S., Menon, V.K., Noronha, R. et al. Callus culture and an unconventional pattern of sporophyte regeneration in Drynaria quercifolia—A medicinal fern. In Vitro Cell.Dev.Biol.-Plant 42, 508–513 (2006). https://doi.org/10.1079/IVP2006810
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1079/IVP2006810