Abstract
Purpose. To investigate the possibility of direct transport of melatonin from the nasal cavity into the cerebrospinal fluid (CSF) after nasal administration in rats and to compare the animal results with a human study.
Methods. Rats (n = 8) were given melatonin both intranasally in one nostril (40 μg/rat) and intravenously by bolus injection (40 μg/rat) into the jugular vein using a Vascular Access Port. Just before and after drug administration, blood and CSF samples were taken and analyzed by HPLC.
Results. Melatonin is quickly absorbed in plasma (T max = 2.5 min) and shows a delayed uptake into CSF (T max = 15 min) after nasal administration. The melatonin concentration-time profiles in plasma and CSF are comparable to those after intravenous delivery. The AUCCSF/AUCplasma ratio after nasal delivery (32.7 ± 6.3%) does not differ from the one after intravenous injection (46.0 ± 10.4%), which indicates that melatonin enters the CSF via the blood circulation across the blood-brain barrier. This demonstrates that there is no additional transport via the nose-CSF pathway. These results resemble the outcome of a human study.
Conclusions. The current results in rats show that there is no additional uptake of melatonin in the CSF after nasal delivery compared to intravenous administration. This is in accordance with the results found in humans, indicating that animal experiments could be predictive for the human situation when studying nose-CSF transport.
Similar content being viewed by others
references
W. M. Pardridge. Knocking on the cerebral door. Odyssey 1:46–51 (1995).
J. Temsamani, J. M. Schermann, A. R. Rees, and M. Kaczorek. Brain drug delivery technologies: novel approaches for transporting therapeutics. PSTT 3:155–162 (2000).
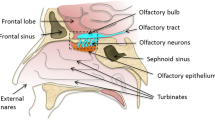
S. Mathison, R. Nagilla, and U. B. Kompella. Nasal route for direct delivery of solutes to the central nervous system: Fact or fiction? J. Drug Targeting 5:415–441 (1998).
L. Illum. Transport of drugs from the nasal cavity to the central nervous system. Eur. J. Pharm. Sci. 11:1–18 (2000).
T. Sakane, S. Yamashita, T. Nadai, and H. Sezaki. Direct drug transport from the nasal cavity to the cerebrospinal fluid. A new strategy for drug delivery to the brain. STP Pharma Sci. 7:98–106 (1997).
K. J. Chou and M. D. Donovan. Distribution of antihistamines into the CSF following intranasal delivery. Biopharm. Drug Dispos. 18:335–346 (1997).
M. Dahlin and E. Björk. Nasal absorption of (S)-UH-301 and its transport into the cerebrospinal fluid of rats. Int. J. Pharm. 195:197–205 (2000).
R. Pietrowsky, L. Claassen, H. Frercks, H. L. Fehm, and J. Born. Time course of intranasally administered cholecystokinin-8 on central nervous effects. Neuropsychobiology 43:254–259 (2001).
R. Pietrowsky, C. Struben, M. Molle, H. L. Fehm, and J. Born. Brain potential changes after intranasal vs intravenous administration of vasopressin: evidence for a direct nose brain pathway for peptide effects in humans. Biol. Psychiatry 39:332–340 (1996).
R. Smolnik, M. Molle, H. L. Fehm, and J. Born. Brain potentials and attention after acute and subchronic intranasal administration of ACTH 4–10 and desacetyl-alpha-MSH in humans. Neuroendocrinology 70:63–72 (1999).
I. Derad, K. Willeke, R. Pietrowsky, J. Born, and H. L. Fehm. Intranasal angiotensin II directly influences central nervous regulation of blood pressure. Am. J. Hypertension 11:971–977 (1998).
W. Kern, J. Born, H. Schreiber, and H. L. Fehm. Central nervous system effects of intranasally administered insulin during euglycemia in men. Diabetes 48:557–563 (1999).
R. Pietrowsky, A. Thiemann, W. Kern, H. L. Fehm, and J. Born. A nose-brain pathway for psychotropic peptides: evidence from a brain evoked potential study with cholecystokinin. Psychoneuroendocrinology 21:559–572 (1996).
F. Qadri, E. Badoer, T. Stadler, and T. Unger. Angiotensin II-induced noradrenaline release from anterior hypothalamus in conscious rats: a brain microdialysis study. Brain Res. 563:137–141 (1991).
A. Veltmar, J. Culman, F. Qadri, W. Rascher, and T. Unger. Involvement of adrenergic and angiotensinergic receptors in the paraventricular nucleus in the angiotensin II-induced vasopressin release. J. Pharmacol. Exp. Ther. 263:1253–1260 (1992).
M. Dahlin, B. Jansson, and E. Björk. Levels of dopamine in blood and brain following nasal administration to rats. Eur. J. Pharm. Sci. 14:75–80 (2001).
T. Sakane, M. Akizuki, M. Yoshida, S. Yamashita, T. Nadai, M. Hashida, and H. Sezaki. Transport of cephalexin to the cerebrospinal fluid directly from the nasal cavity. J. Pharm. Pharmacol. 43:449–451 (1991).
T. Yajima, K. Juni, M. Saneyoshi, T. Hasegawa, and T. Kawaguchi. Direct transport of 2′,3′-didehydro-3′-deoxythymidine (D4T) and its ester derivatives to the cerebrospinal fluid via the nasal mucous membrane in rats. Biol. Pharm. Bull. 21:272–277 (1998).
T. Seki, N. Sato, T. Hasegawa, T. Kawaguchi, and K. Juni. Nasal absorption of zidovudine and its transport to cerebrospinal fluid in rats. Biol. Pharm. Bull. 17:1135–1137 (1994).
H. Tjäve and I. Henriksson. Uptake of metals in the brain via olfactory pathways. Neurotoxicology 20:181–195 (1999).
A. J. Martinez, R. J. Duma, E. C. Nelson, and F. L. Moretta. Experimental naegleria meningoencephalitis in mice. Penetration of the olfactory mucosal epithelium by naegleria and pathologic changes produced: a light and electron microscope study. Lab. Invest. 29:121–133 (1973).
K. L. Jarolim, J. K. McCosh, M. J. Howard, and D. T. John. A light microscopy study of the migration of naegleria fowleri from the nasal submucosa to the central nervous system during the early stage of primary amebic meningoencephalitis in mice. J. Parasitol. 86:50–55 (2000).
T. C. Anand Kumar, G. F. X. David, B. Umberkoman, and K. D. Saini. Uptake of radioactivity by body fluids and tissues in rhesus monkeys after intravenous injection or intranasal spray of tritium-labelled oestradiol and progesterone. Curr. Sci. 43:435–439 (1974).
S. Gizurarson and E. Bechgaard. Intranasal administration of insulin to humans. Diabetes Res. Clin. Pract. 12:71–84 (1991).
X. F. Liu, J. R. Fawcett, R. G. Thorne, T. A. DeFor, and W. H. Frey. Intranasal administration of insulin-like growth factor-I bypasses the blood-brain barrier and protects against focal cerebral ischemic damage. J. Neurol. Sci. 187:91–97 (2001).
P. Merkus, H. J. Guchelaar, D. A. Bosch, and F. W. H. M. Merkus. Direct access of drugs to the human brain after intranasal drug administration? Neurology 60:1669–1671 (2003).
L. Kikwai, N. Kanikkannan, R. J. Babu, and M. Singh. Effect of vehicles on the transdermal delivery of melatonin across porcine skin in vitro. J. Control. Rel. 83:307–311 (2002).
F. W. H. M. Merkus. Nasal melatonin compositions. US Patent No. 6 007 834 (1999).
M. P. Van den Berg, S. G. Romeijn, J. C. Verhoef, and F. W. H. M. Merkus. Serial cerebrospinal fluid sampling in a rat model to study drug uptake from the nasal cavity. J. Neurosci. Meth. 116:99–107 (2002).
M. P. Van den Berg, J. C. Verhoef, S. G. Romeijn, and F. W. H. M. Merkus. Uptake of hydrocortisone into the cerebrospinal fluid of rats: comparison of intranasal and intravenous administration in the same animal. STP Pharma Sci. 12:251–255 (2002).
J. Sastre Toraño, P. van Rijn-Bikker, P. Merkus, and H. J. Guchelaar. Quantitative determination of melatonin in human plasma and cerebrospinal fluid with high-performance liquid chromatography and fluorescence detection. Biomed. Chromatogr. 14:306–310 (2000).
M. A. Hussain, D. Rakestraw, S. Rowe, and B. J. Aungst. Nasal administration of a cognition enhancer provides improved bioavailability but not enhanced brain delivery. J. Pharm. Sci. 79:771–772 (1990).
M. P. Van den Berg, P. Merkus, S. G. Romeijn, J. C. Verhoef, and F. W. H. M. Merkus. Hydroxocobalamin uptake into the cerebrospinal fluid after nasal and intravenous delivery in rats and humans. J. Drug Target. 11:325–331 (2003).
J. M. DeSesso. The relevance to humans of animal models for inhalation studies of cancer in the nose and upper airways. Qual. Assur. 2:213–231 (1993).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
van den Berg, M.P., Merkus, P., Romeijn, S.G. et al. Uptake of Melatonin into the Cerebrospinal Fluid After Nasal and Intravenous Delivery: Studies in Rats and Comparison with a Human Study. Pharm Res 21, 799–802 (2004). https://doi.org/10.1023/B:PHAM.0000026431.55383.69
Issue Date:
DOI: https://doi.org/10.1023/B:PHAM.0000026431.55383.69