Abstract
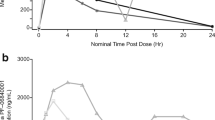
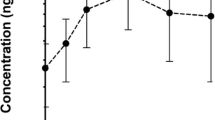
Objectives: CCI-779 is an ester of the immunosuppressive agent sirolimus (rapamycin) that causes cell-cycle arrest at G1 via inhibition of key signaling pathways resulting in inhibition of RNA translation. Antitumor activity has been demonstrated using cell lines and animal models of malignant glioma. Patients receiving enzyme-inducing anti-epileptic drugs (EIAEDs) can have altered metabolism of drugs like CCI-779 that are metabolized through the hepatic cytochrome P450 enzyme system. The objectives of this study were to determine the pharmacokinetic profile and the maximum tolerated dose of CCI-779 in patients with recurrent malignant gliioma taking EIAEDs. Study design: The starting dose of CCI-779 was 250 mg intravenously (IV) administered weekly on a continuous basis. Standard dose escalation was performed until the maximum tolerated dose was established. Toxicity was assessed using the National Cancer Institute common toxicity criteria. Results: Two of 6 patients treated at the second dose level of 330 mg sustained a dose-limiting toxicity: grade III stomatitis, grade 3 hypercholesterolemia, or grade 4 hypertriglyceridemia. The maximum tolerated dose was reached at 250 mg IV. Pharmacokinetic profiles were similar to those previously described, but the area under the whole blood concentration-time curve of rapamycin was 1.6 fold lower for patients on EIAEDs. Conclusions: The recommended phase II dose of CCI 779 for patients on enzyme-inducing antiepileptic drugs is 250 mg IV weekly. A phase II study is ongoing to determine the efficacy of this agent.
Similar content being viewed by others
References
Wong ET, Hess KR, Gleason MJ, Jaeckle KA, Kyritsis AP, Prados MD, Levin VA, Yung WK: Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol 17: 2572, 1999
Knobbe CB, Merlo A, Reifenberger G: Pten signaling in gliomas. Neuro-oncol 4: 196–211, 2002
Sasaki H, Zlatescu MC, Betensky RA, Ino Y, Cairncross JG, Louis DN: PTEN is a target of chromosome 10q loss in anaplastic oligodendrogliomas and PTEN alterations are associated with poor prognosis. Am J Pathol 159: 359–367, 2001
Sano T, Lin H, Chen X, Langford LA, Koul D, Bondy ML, Hess KR, Myers JN, Hong YK, Yung WK, Steck PA: Differential expression of MMAC/PTEN in glioblastoma multiforme: Relationship to localization and prognosis. Cancer Res 59: 1820–1824, 1999
Smith JS, Tachibana I, Passe SM, Huntley BK, Borell TJ, Iturria N, O'Fallon JR, Schaefer PL, Scheithauer BW, James CD, Buckner JC, Jenkins RB: PTEN mutation, EGFR amplification, and outcome in patients with anaplastic astrocytoma and glioblastoma multiforme. J Natl Cancer Inst 93: 1246–1256, 2001
Hidalgo M, Rowinsky EK: The rapamycin-sensitive signal transduction pathway as a target for cancer therapy. Oncogene 19: 6680–6686, 2000
Chen J, Fang Y: A novel pathway regulating the mammalian target of rapamycin (mTOR) signaling. Biochem Pharmacol 64: 1071–1077, 2002
Schmelzle T, Hall MN: TOR, a central controller of cell growth. Cell 103: 253–262, 2000
Geoerger B, Kerr K, Tang CB, Fung KM, Powell B, Sutton LN, Phillips PC, Janss AJ: Antitumor activity of the rapamycin analog CCI-779 in human primitive neuroectodermal tumor/medulloblastoma models as single agent and in combination chemotherapy. Cancer Res 61: 1527–1532, 2001
Neshat MS, Mellinghoff IK, Tran C, Stiles B, Thomas G, Petersen R, Frost P, Gibbons JJ, Wu H, Sawyers CL, Podsypanina K, Lee RT, Politis C, Hennessy I, Crane A, Puc J, Neshat M, Wang H, Yang L, Gibbons J, Dreisbach V, Blenis J, Gaciong Z, Fisher P, Sawyers C, Hedrick-Ellenson L, Parsons R: Enhanced sensitivity of PTEN-deficient tumors to inhibition of FRAP/mTOR An inhibitor of mTOR reduces neoplasia and normalizes p70/S6 kinase activity in Pten ± mice. Proc Natl Acad Sci USA 98: 10314–10319, 2001
Podsypanina K, Lee RT, Politis C, Hennessy I, Crane A, Puc J, Neshat M, Wang H, Yang L, Gibbons J, Frost P, Dreisbach V, Blenis J, Gaciong Z, Fisher P, Sawyers C, Hedrick-Ellenson L, Parsons R: An inhibitor of mTOR reduces neoplasia and normalizes p70/S6 kinase activity in Pten ± mice. Proc Natl Acad Sci USA 98: 10320–10325, 2001
Huang S, Houghton PJ: Targeting mTOR signaling for cancer therapy. Curr Opin Pharmacol 3: 371–377, 2003
Macdonald DR, Cascino TL, Schold SC, Jr., Cairncross JG: Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 8: 1277–1280, 1990
Powis G, Bonjouklian R, Berggren MM, Gallegos A, Abraham R, Ashendel C, Zalkow L, Matter WF, Dodge J, Grindey G, Vlahos CJ: Wortmannin, a potent and selective inhibitor of phosphatidylinositol-3-kinase. Cancer Res 54: 2419–2423, 1994
Vlahos CJ, Matter WF, Hui KY, Brown RF: A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002). J Biol Chem 269: 5241–5248, 1994
Beuvink I, O'Reilly T, Zumstein S, Zilbermann F, Sedrani R, Kozma S, Thomas G, Lans H: Antitumor activity of RAD001, 435 an orally active rapamycin derivative. Proceedings of American Association of Cancer Research 42: 366, 2001
Chang SM, Kuhn JG, Rizzo J, Robins HI, Schold SC, Jr., Spence AM, Berger MS, Mehta MP, Bozik ME, Pollack I, Gilbert M, Fulton D, Rankin C, Malec M, Prados MD: Phase I study of paclitaxel in patients with recurrent malignant glioma: A North American Brain Tumor Consortium report. J Clin Oncol 16: 2188–2194, 1998
Prados M, Kuhn J, Yung W, Robins H, Fink K, Greenberg H, Junck L, Cloughesy T, Chang S, Fine H, Schiff D, Nicholas M: A phase I study of CPT-11 given every 3 weeks to patients with recurrent malignant glioma. A North American Consortium study. Proc Am Soc Clin Oncol 19: 162,a, 2000
Cloughesy T, Kuhn J, Wen P, Chang S, Schiff D, Greenberg H, Junck L, Robins I, De Angelis L, Raizer J, Hess K, Prados M: Phase II trial of R115777 (Zarnestra) in patients with recurrent glioma not taking enzyme-inducing antiepileptic drugs (EIAED): A North American Brain Tumor Consortium (NABTC) report. Proc Am Soc Clin Oncol21: 80a, 2002
Raymond E, Alexandre J, Depenbrock H, Mekhaldi S, Angevin E, Hanauske A, Baudin E, Escudier B, Frisch J, Boni J, Armand JP: CCI-779: A rapamycin analog with antitumor activity: A phase I study utilizing a weekly schedule (Abstract). Proc Am Soc Clin Oncol 19: 187a, 2000
Atkins MB, Hidalgo M, Stadler W, Logan T, Dutcher JP, Hudes G, Park Y, Marshall B, Boni J, Dukart G: A randomized double-blind phase II study of intravenous CCI-779 administrated weekly to patients with advanced renal cell carcinoma (Abstract). Proc Am Soc Clin Oncol 21: 10a, 2002
Chan S, Johnston S, Scheulen ME, Mross K, Morant R, Lahr A, Feussner A, Berger M, Kirsch T: First report: A phase 2 study of the safety and activity of CCI-779 for patients with locally advanced or metastatic breast cancer failing prior chemotherapy (Abstract). Proc Am Soc Clin Oncol 21: 44a, 2002
Brattstrom C, Sawe J, Jansson B, Lonnebo A, Nordin J, Zimmerman JJ, Burke JT, Groth CG: Pharmacokinetics and safety of single oral doses of sirolimus (rapamycin) in healthy male volunteers. Ther Drug Monit 22: 537–544, 2000
Eshleman JS, Carlson BL, Mladek AC, Kastner BD, Shide KL, Sarkaria JN: Inhibition of the mammalian target of rapamycin sensitizes U87 xenografts to fractionated radiation therapy. Cancer Res 62: 7291–7297, 2002
Peralba JM, DeGraffenried L, Friedrichs W, Fulcher L, Grunwald V, Weiss G, Hidalgo M: Pharmacodynamic evaluation of CCI-779, an inhibitor of mTOR, in cancer patients. Clin Cancer Res 9: 2887–2892, 2003
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Chang, S.M., Kuhn, J., Wen, P. et al. Phase I/pharmacokinetic study of CCI-779 in patients with recurrent malignant glioma on enzyme-inducing antiepileptic drugs. Invest New Drugs 22, 427–435 (2004). https://doi.org/10.1023/B:DRUG.0000036685.72140.03
Issue Date:
DOI: https://doi.org/10.1023/B:DRUG.0000036685.72140.03