Abstract
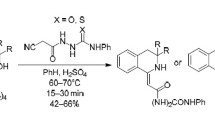
The reaction of (arylhydrazono)cyanothioacetamides with chloro ketones and phenacyl bromides was studied. The products are either thiazoles or 4,5-dihydrothiophenes, depending on the starting thioamides and halo ketones.
Similar content being viewed by others
REFERENCES
V. P. Litvinov, Usp. Khim., 68, 817 (1999).
H. Tokuyama,T. Amashita,M. T. Reding,Y. Koburagi, andT. Fukuyama, J. Am. Chem. Soc., 121, 3791 (1999).
H. Takahata andE. Yamazaki, Heterocycles, 27, 1953 (1988).
R. G. Dubenko,I. M. Bazavova, andE. F. Gorbenko, Zh. Org. Khim., 20, 577 (1984).
N. P. Belskaia,E. E. Zvereva,W. Dehaen, andV. A. Bakulev, J. Chem. Res. (M), 1367 (2000).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Paramonov, I.V., Belskaia, N.P. & Bakulev, V.A. Reaction of (Arylhydrazono)cyanothioacetamides with Halo Ketones. Chemistry of Heterocyclic Compounds 39, 1385–1395 (2003). https://doi.org/10.1023/B:COHC.0000010657.14733.8e
Issue Date:
DOI: https://doi.org/10.1023/B:COHC.0000010657.14733.8e