Abstract
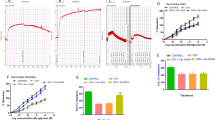
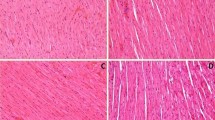
Previous investigations have shown that a single dose of low-density lipoprotein (LDL) injection causes a marked decrease in endothelium-dependent relaxation, and that endothelial dysfunction aggravates myocardial injury due to ischemia-reperfusion in atherosclerotic animals. Monophosphoryl lipid A-induced delayed preconditioning preserves the ischemic myocardium and endothelial cells. The objective of the present study was to examine the effect of monophosphoryl lipid A on endothelial function and ischemia-reperfusion-induced myocardial injury in the rats treated with LDL. A single injection of native LDL (4 mg/kg, i.v.) caused a significant decrease in vasodilator responses to acetylcholine and an increase in the serum level of asymmetric dimethylarginine, the endogenous nitric oxide synthase inhibitor. Pretreatment with monophosphoryl lipid A (300 or 450 μg/kg, i.p.) significantly improved endothelium-dependent relaxation and decreased concentrations of asymmetric dimethylarginine, and the effects of monophosphoryl lipid A were attenuated by L-nitro-arginine-methylester, but not by aminoguanidine. LDL treatment only aggravated the decreased coronary flows but did not affect cardiac dysfunction due to ischemia-reperfusion in the rats treated with LDL. Pretreatment with monophosphoryl lipid A significantly improved cardiac dysfunction by ischemia-reperfusion in the rats treated with or without LDL, an effect that was abolished by aminoguanidine. The present study suggests that: (1) a single injection of native LDL causes a decrease in endothelium-dependent relaxation, and aggravates the decrease of coronary flow due to ischemia-reperfusion, (2) pretreatment with monophosphoryl lipid A protects the myocardium against ischemia-reperfusion in the rats treated with or without native LDL, and (3) the protective effect of monophosphoryl lipid A on endothelial cells is related to reduction of asymmetric dimethylarginine level.
Similar content being viewed by others
References
Chang MY, Lees AM, Lees RS. Low-density lipoprotein modification and arterial wall accumulation in a rabbit model of atherosclerosis. Biochemistry 1993;32:8518-8524.
Hein TW, Kuo L. LDLs impair vasomotor function of the coronary microcirculation: Role of superoxide anions. Circ Res 1998;83:404-414.
Okuno S, Thoren P, Lowbeer C, Valen G. The role of nitric oxide in ischaemia/reperfusion injury of isolated hearts from severely atherosclerotic mice. Life Sci 2001;69:2067-2080.
Yu XJ, Li YJ, Xiong Y. Increase of an endogenous inhibitor of nitric oxide synthesis in serum of high cholesterol fed rabbits. Life Sci 1994;54:753-758.
Boger RH, Bode-Böger SM, Szuba A, et al. Asymmetric dimethylarginine: A novel risk factor for endothelial dysfunction: Its role in hypercholesterolemia. Circulation 1998;98:1842-1847.
Jiang JL, LiNs NS, Li YJ, Deng HW. Probucol preserves endothelial function by reduction of the endogenous nitric oxide synthase inhibitor level. Br J Pharmacol 2002;135:1175-1182.
Yao Z, Elliott GT, Gross GJ. Monophosphoryl lipid A preserves myocardial contractile function following multiple, brief periods of coronary occlusion in dogs. Pharmacology 1995;51:152-159.
Baxter GF, Goodwin RW, Wright MJ, Kerac M, Heads RJ, Yellon DM. Myocardial protection after monophosphoryl lipid A: Studies of delayed anti-ischaemic properties in rabbit heart. Br J Pharmacol 1996;117:1685-1692.
Yoshida K, Maaieh MM, Shipley JB, et al. Monophosphoryl lipid A induces pharmacologic' preconditioning' in rabbit hearts without concomitant expression of 70-kDa heat shock protein. Mol Cell Biochem 1996;156:1-8.
Zhao L, Weber PA, Smith JR, Comerford ML, Elliott GT. Role of inducible nitric oxide synthase in pharmacological “preconditioning” with monophosphoryl lipid A. J Mol Cell Cardiol 1997;29:1567-1576.
Elliott GT.Monophosphoryl lipid A induces delayed preconditioning against cardiac ischemia-reperfusion injury.JMol Cell Cardiol 1998;30:3-17.
Peng J, Lu R, Ye F, Deng HW, Li YJ. The heme oxygenase-1 pathway is involved in calcitonin gene-related peptidemediated delayed cardioprotection induced by monophosphoryl lipid A in rats. Regul Pept 2002;103:1-7.
Richard V, Kaeffer N, Tron C, Thuillez C. Ischemic preconditioning protects against coronary endothelial dysfunction induced by ischemia and reperfusion. Circulation 1994;89:1254-1261.
Kaeffer N, Richard V, Francois A, Lallemand F, Henry JP, Thuillez C. Preconditioning prevents chronic reperfusioninduced coronary endothelial dysfunction in rats. Am J Physiol 1996;271(3 Pt 2):H842-H849.
Kaeffer N, Richard V, Thuillez C. Delayed coronary endothelial protection 24 hours after preconditioning: Role of free radicals. Circulation 1997;96:2311-2316.
Richard V, Danielou E, Kaeffer N, Thuillez C. Delayed endothelial protective effects of monophosphoryl lipid A after myocardial ischemia and reperfusion in rats. JMol Cell Cardiol 1999;31:1117-1123.
Salkowski CA, Detore G, Franks A, Falk MC, Vogel SN. Pulmonary and hepatic gene expression following cecal ligation and puncture: Monophosphoryl lipid A prophylaxis attenuates sepsis-induced cytokine and chemokine expression and neutrophil infiltration. Infect Immun 1998;66:3569-3578.
Gustafson GL, Rhodes MJ, Hegel T. Monophosphoryl lipid A as a prophylactic for sepsis and septic shock. Prog Clin Biol Res 1995;392:567-579.
Ito A, Tsao PS, Adimoolam S, Kimoto M, Ogawa T, Cooke JP. Novel mechanism for endothelial dysfunction: Dysregulation of dimethylarginine dimethylaminohydrolase. Circulation 1999;99:3092-3095.
Chen BM, Xia LW, Zhao RQ. Determination of N(G), N(G)-dimethylarginine in human plasma by high-performance liquid Chromatography. J Chromatogr B Biomed Sci Appl 1997;692:467-471.
Kugiyama K, Kerns SA, Morrisett JD, Roberts R, Henry PD. Impairment of endothelium-dependent arterial relaxation by lysolecithin in modified low-density lipoproteins. Nature 1990;344:160-162.
Frostegard J, Nilsson J, Haegerstrand A, Hamsten A, Wigzell H, Gidlund M. Oxidized low density lipoprotein induces differentiation and adhesion of human monocytes and the monocytic cell line U937. Proc Natl Acad Sci USA 1990;87:904-908.
Frostegard J, Haegerstrand A, Gidlund M, Nilsson J. Biologically modified LDL increases the adhesive properties of endothelial cells. Atherosclerosis 1991;90:119-126.
Koba S, Pakala R, Watanabe T, Katagiri T, Benedict CR. Synergistic interaction between thromboxaneA2 and mildly oxidized low density lipoproteins on vascular smooth muscle cell proliferation. Prostaglandins Leukot Essent Fatty Acids 2000;63:329-335.
Calara F, Dimayuga P, Niemann A, et al. An animal model to study local oxidation of LDL and its biological effects in the arterial wall. Arterioscler Thromb Vasc Biol 1998;18:884-893.
Vallance P, Leone A, Calver A, Collier J, Moncada S. Accumulation of an endogenous inhibitor of nitric oxide synthesis in chronic renal failure. Lancet 1992;339:572-575.
Kurose I, Wolf R, Grisham MB, Granger DN. Effects of an endogenous inhibitor of nitric oxide synthesis on postcapillary venules. Am J Physiol 1995;268:H2224-H2231.
Usui M, Matsuoka H, Miyazaki H, Ueda S, Okuda S, Imaizumi T. Increased endogenous nitric oxide synthase inhibitor in patient with congestive heart failure. Life Sci 1998;62:2425-2430.
Surdacki A, Nowicki M, Sandmann J, et al. Reduced urinary excretion of nitric oxide metabolites and increased plasma levels of asymmetric dimethylarginine in men with essential hypertension. J Cardiovasc Pharmacol 1999;33:652-658.
Böger RH, Sydow K, Borlak J, et al. LDL cholesterol upregulates synthesis of asymmetrical dimethylarginine in human endothelial cells: Involvement of S-adenosylmethioninedependent methyltransferases. Circ Res 2000;87:99-105.
Leiper JM, Santa, Maria J, et al. Identification of two human dimethylarginine dimethylaminohydrolases with distinct tissue distributions and homology with microbial arginine deiminases. Biochem J 1999;343(Pt 1):209-214.
Tran CT, Fox MF, Vallance P, Leiper JM. Chromosomal localization, gene structure, and expression pattern of DDAH1: Comparison with DDAH2 and implications for evolutionary origins. Genomics 2000;68:101-105.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Zhu, HQ., Jiang, JL., Lu, R. et al. The Protective Effects of Monophosphoryl Lipid A on the Ischemic Myocardium and Endothelium in Rats. Cardiovasc Drugs Ther 17, 311–318 (2003). https://doi.org/10.1023/A:1027335321530
Issue Date:
DOI: https://doi.org/10.1023/A:1027335321530