Abstract
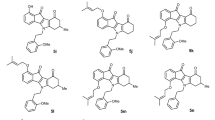
The development of multidrug-resistance (MDR) in neoplastic cells is often responsible for the therapy failure and poor outcome of a number of human cancers. MDR may be associated with the expression of the multidrug transporter glycoprotein p170, encoded by the MDR1 gene, which acts as an ATP-dependent efflux pump by reducing the intracellular accumulation of some cytotoxic agents. A variety of iminodibenzyl and phenothiazine derivatives, characterized by the presence of a bicyclic, strongly basic, and highly lipophilic quinolizidine nucleus, were synthesized to investigate their ability to modulate the MDR phenotype. A set of 10 of them (named 1–10), bearing quinolizidine moiety linked through different connecting chains, were tested as chemoresistance-reversing agents on doxorubicin-resistant ovarian cancer cells (A2780-DX3). A 51-fold resistance to doxorubicin was reported in the A2780-DX3 compared to the parental sensitive A2780 WT with mean IC50 values of 0.02 and 1.02 μM, respectively. Moreover, overexpression of the glycoprotein p170 in the resistant cell line was detected by Western blot analysis. By cytotoxicity assays and time-course experiments, different treatment schedules with resistance modulators (including clomipramine as reference drug) and doxorubicin were taken into account. The 16 h exposure of cells to 1 μM of modulator before doxorubicin demonstrated to be superior in sensitizing the resistant cell line. In particular, compounds 8, 7, 10, and 4 increasingly potentiated doxorubicin cytotoxicity, up to 5.6-fold in A2780-DX3 cells. The present results suggest promising indications for further development of these compounds as chemosensitizing drugs.
Similar content being viewed by others
References
Ford JM, Hait WN: Pharmacology of drugs that alter multidrug resistance in cancer. Pharmacol Rev 42: 155–199, 1990
Ling V: Multidrug resistance: molecular mechanisms and clinical relevance. Cancer Chemother Pharmacol 40(suppl.): S3–S8, 1997
Sikic BJ, Fisher GA, Lum BL, Halsey J, Beketic-Oreskovic L, Chen G: Modulation and prevention of multidrug resistance by inhibitors of P-glycoprotein. Cancer Chemother Pharmacol 40(suppl.): S13–S19, 1997
Geney R, Ungureanu M, Li D, Ojima I: Overcoming multidrug resistance in taxane chemotherapy. Clin Chem Lab Med 40: 918–925, 2002
Wiese M, Pajeva IK: Structure-activity relationship of multidrug resistance reversers. Curr Med Chem 8: 685–713, 2001
Teodori E, Dei S, Scapecchi S, Gualtieri F: The medicinal chemistry of multidrug resistance (MDR) reversing drugs. Farmaco 57: 385–415, 2002
Ford MJ, Prozialeck WC, Hait WN: Structural features determining activity of phenothiazines and related drugs for inhibition of cell growth and reversal of multidrug resistance. Mol Pharmacol 35: 105–115, 1989
Ford JM, Bruggeman EP, Pastan I, Gottesman MM, Hait WN: Cellular and biochemical characterization of thioxanthenes for reversal of multidrug resistance in human and murine cell lines. Cancer Res 50: 1748–1756, 1990
Ramu A, Ramu N: Reversal of multidrug resistance by phenothiazines and structurally related compounds. Cancer Chemother Pharmacol 30: 165–173, 1992
Pajeva IK, Wiese M: QSAR and molecular modelling of catamphiphilic drugs able to modulate multidrug resistance in tumors. Quant Struct Act Relat 16: 1–10, 1997
Pajeva IK, Wiese M: Molecular modeling of phenothiazines and related drugs as multidrug resistance modifier: a comparative molecular field analysis study. J Med Chem 41: 1815–1826, 1998
Sparatore F, Boido V: Derivati di amminoalcoli e diammine naturali di interesse farmacologico. Nota III. Sintesi di derivati lupinilici di sistemi eterociclici trianellari. Ann Chim 54: 591–601, 1964
Boido V, Sparatore A: Chinolizidinil derivati di sistemi triciclici. Farmaco 37: 63–73, 1982
Sparatore A, Sparatore F: Preparation and pharmacological activities of 10–homolupinanoyl-2–R-phenothiazines. Farmaco 49: 5–17, 1994
Novelli F, Sparatore A, Tasso B, Sparatore F: Quinolizidinyl derivatives of 5,11–dihydro-6H-pyrido(2,3–b(-(1,4( benzodiazepin-6–one as ligands for muscarinic receptors. Bioorg Med Chem Lett 9: 3031–3034, 1999
Tasso B, Sparatore A, Sparatore F: N-Homolupinanoyl-and N-(ω-lupinylthio)alkanoyl derivatives of some tricyclic systems as ligands for muscarinic M1 and M2 receptor subtypes. Farmaco, in press, 2003
Mazzoni A, Trave F: Cytoplasmic membrane cholesterol and doxorubicin cytotoxicity in drug-sensitive and multidrug-resistant human ovarian cancer cells. Oncol Res 5: 75–82, 1993
Häfliger F, Schindler W: N-Acyl derivatives of iminodibenzyl. US2, 666,051. Chem Abstr 49: 9041f, 1955
Ekstrand T: Phenothiazine derivatives of pharmacological interest. Acta Chem Scand 3: 302–303, 1949
Société des usines chimiques Rhone-Poulenc. Phenothiazine derivatives. Brit 740, 932. Chem Abstr 51: 500i, 1957
Kazutoshi F, Atsuo S, Kazuo S, Kazuo O: 10–Chloroacetyl phenothiazine. Japan 74 29,19. Chem Abstr 82: 140161w, 1975
Toldy L, Toth I, Fekete M, Borsy J: Phenothiazine derivatives. VI. Experiments for the preparation of phenothiazines with selective coronary dilator activity. Acta Chim Acad Sci Hung 44: 301–325, 1965
Novelli F, Sparatore F: Thiolupinine and some derivatives of pharmacological interest. Farmaco 48: 1021–1049, 1993
Domenjoz R, Theobald W: Pharmacologie des Tofranil (N-(3–dymethylaminopropyl)-iminodibenzyl hydrochloride). Arch Int Pharmacodyn 120: 450–489, 1959
Tobe A, Yoshida Y, Ikoma H, Tonomura S, Kikumoto R: Pharmacological evaluation of 2–(4–methylaminobuthoxy)diphenylmethane hydrochloride, a new psychotropic drug with antidepressant activity. Arzneim Forsch 31: 1278–1285, 1981
Guan J, Kyle DE, Gerena L, Zhang Q, Milhous WK, Lin AJ: Design, synthesis and evaluation of new chemosensitizers in multi-drug resistant Plasmodium falciparum. J Med Chem 45: 2741–2748, 2002
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Barbieri, F., Alama, A., Tasso, B. et al. Quinolizidinyl derivatives of iminodibenzyl and phenothiazine as multidrug resistance modulators in ovarian cancer cells. Invest New Drugs 21, 413–420 (2003). https://doi.org/10.1023/A:1026295017158
Issue Date:
DOI: https://doi.org/10.1023/A:1026295017158