Abstract
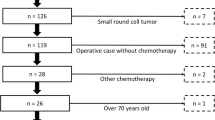
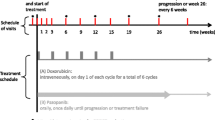
Background: The number of effective cytotoxic agents for the treatment of patients with metastatic soft tissue sarcoma (STS) is limited, especially when patients have failed anthracyline-based chemotherapy. Patients and methods: Between 1999 and 2000 a total of 16 patients with histologically proven STS progressing during or after first-line anthracycline-based chemotherapy were entered into this open-label, noncomparative study. Topotecan was administered as a 30-min infusion at a dosage of 1.5 mg/m2 on five consecutive days every 3 weeks. All patients had received an anthracycline- or ifosfamide-based first-line chemotherapy. Results: None of the 16 included patients achieved an objective response to topotecan. Six patients achieved stable disease (38%), lasting for at least 6 weeks in four patients (25%) and for less than 6 weeks in two patients (13%). Ten patients (62%) had progressive disease. The median time to progression was 79 days calculated from the start of topotecan therapy (range, 28–230). The treatment was well tolerated; however, both anemia and thrombopenia grade III/IV occurred in 25% of the patients as well as severe neutropenia in 69% of the patients. Nonhematologic toxicities grade III/IV such as diarrhea and severe bleeding occurred only in one patient each (6%). Discussion: Topotecan is well tolerated in anthracycline-resistant patients with metastatic STS, but no objective response has been observed in this trial.
Similar content being viewed by others
References
Patel SR: Systemic chemotherapy, In Pollock RE (ed) Soft tissue sarcomas, Hamilton, Ontario, Canada, 2002, pp. 323–328
Benjamin RS, Legha SS, Patel SR, Nicaise C: Single-agent ifosfamide studies in sarcomas of soft tissue and bone: the M.D. Anderson experience. Cancer Chemother Pharmacol 31(suppl 2): S174–S179, 1993
Borden EC, Amato DA, Rosenbaum C, Enterline HT, Shiraki MJ, Creech RH, Lerner HJ, Carbone PP: Randomized comparison of three adriamycin regimens for metastatic soft tissue sarcomas. J Clin Oncol 5: 840–850, 1987
Lopez M, Vici P, Di Lauro L, Carpano S: Increasing single epirubicin doses in advanced soft tissue sarcomas. J Clin Oncol 20: 1329–1334, 2002
Cure H, Krakowski I, Adenis A, Tubiana N, Kerbrat P, Roche H, Chevallier B, Lentz MA, Fumoleau P, Chollet P: Results of a phase II trial with second-line cystemustine at 60 mg/m2 in advanced soft tissue sarcoma: a trial of the EORTC Early Clinical Studies Group. Eur J Cancer 34: 422–423, 1998
Hartmann JT, Oechsle K, Mayer F, Kanz L, Bokemeyer C: Phase II trial of trofosfamide in patients with advanced pretreated soft tissue sarcomas. Anticancer Res, 2003 Mar–Apr; 23(2C): 1899–1901. PM1D: 12820475[Pubmed-indexed for MEDLINE].
Spath-Schwalbe E, Genvresse I, Koschuth A, Dietzmann A, Grunewald R, Possinger K: Phase II trial of gemcitabine in patients with pretreated advanced soft tissue sarcomas. Anticancer Drugs 11: 325–329, 2000
Papai Z, Bodoky G, Szanto J, Poller I, Rahoty P, Eckhardt S, Lang I, Szendroi M: The efficacy of a combination of etoposide, ifosfamide, and cisplatin in the treatment of patients with soft tissue sarcoma. Cancer 89: 177–180, 2000
O'Reilly S, Rowinsky EK, Slichenmyer W, Donehower RC, Forastiere AA, Ettinger DS, Chen TL, Sartorius S, Grochow LB: Phase I and pharmacologic study of topotecan in patients with impaired renal function. J Clin Oncol 14: 3062–3073, 1996
Bramwell VH, Eisenhauer EA, Blackstein M, Boos G, Knowling M, Jolivet J, Bogues W: Phase II study of topotecan (NSC 609 699) in patients with recurrent or metastatic soft tissue sarcoma. Ann Oncol 6: 847–849, 1995
Saylors RL, III, Stine KC, Sullivan J, Kepner JL, Wall DA, Bernstein ML, Harris MB, Hayashi R, Vietti TJ: Cyclophosphamide plus topotecan in children with recurrent or refractory solid tumors: a pediatric oncology group phase II study. J Clin Oncol 19: 3463–3469, 2001
WHO (ed): International classification of diseases, 10th edn (ICD-10). Geneva, 1996
Fleming TR: One-sample multiple testing procedure for phase II clinical trials. Biometrics 38: 143–151, 1982
Kaplan EL, Maier P: Non-parametric estimation from incomplete observations. J Am Stat Assoc 53: 457–481, 1958
Bokemeyer C, Franzke A, Hartmann JT, Schober C, Arseniev L, Metzner B, Link H, Kanz L, Schmoll HJ: A phase I/II study of sequential, dose-escalated, high dose ifosfamide plus doxorubicin with peripheral blood stem cell support for the treatment of patients with advanced soft tissue sarcomas. Cancer 80: 1221–1227, 1997
Frustaci S, Buonadonna A, Galligioni E, Favaro D, De Paoli A, Lo RG, Sorio R, Tumolo S, Monfardini S: Increasing 4′-epidoxorubicin and fixed ifosfamide doses plus granulocyte-macrophage colony-stimulating factor in advanced soft tissue sarcomas: a pilot study. J Clin Oncol 15: 1418–1426, 1997
Reichardt P, Tilgner J, Hohenberger P, Dorken B: Dose-intensive chemotherapy with ifosfamide, epirubicin, and filgrastim for adult patients with metastatic or locally advanced soft tissue sarcoma: a phase II study. J Clin Oncol 16: 1438–1443, 1998
Steward WP, Verweij J, Somers R, Spooner D, Kerbrat P, Clavel M, Crowther D, Rouesse J, Tursz T, Tueni E: Granulocyte-macrophage colony-stimulating factor allows safe escalation of dose-intensity of chemotherapy in metastatic adult soft tissue sarcomas: a study of the European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group. J Clin Oncol 11: 15–21, 1993
Patel SR, Vadhan-Raj S, Burgess MA, Plager C, Papadopolous N, Jenkins J, Benjamin RS: Results of two consecutive trials of dose-intensive chemotherapy with doxorubicin and ifosfamide in patients with sarcomas. Am J Clin Oncol 21: 317–321, 1998
Balcerzak SP, Benedetti J, Weiss GR, Natale RB: A phase II trial of paclitaxel in patients with advanced soft tissue sarcomas. A Southwest Oncology Group study. Cancer 76: 2248–2252, 1995
Svancarova L, Blay JY, Judson IR, van Hoesel QG, van Oosterom AT, le Cesne A, Keizer HJ, Hermans C, Van Glabbeke M, Verweij J, Hogendoorn PC, Nielsen OS: Gemcitabine in advanced adult soft-tissue sarcomas. A phase II study of the EORTC Soft Tissue and Bone Sarcoma Group. Eur J Cancer 38: 556–559, 2002
Okuno S, Edmonson J, Mahoney M, Buckner JC, Frytak S, Galanis E: Phase II trial of gemcitabine in advanced sarcomas. Cancer 94: 3225–3229, 2002
Patel SR, Gandhi V, Jenkins J, Papadopolous N, Burgess MA, Plager C, Plunkett W, Benjamin RS: Phase II clinical investigation of gemcitabine in advanced soft tissue sarcomas and window evaluation of dose rate on gemcitabine triphosphate accumulation. J Clin Oncol 19: 3483–3489, 2001
Hensley ML, Maki R, Venkatraman E, Geller G, Lovegren M, Aghajanian C, Sabbatini P, Tong W, Barakat R, Spriggs DR: Gemcitabine and docetaxel in patients with unresectable leiomyosarcoma: results of a phase II trial. J Clin Oncol 20: 2824–2831, 2002
van Hoesel QG, Verweij J, Catimel G, Clavel M, Kerbrat P, van Oosterom AT, Kerger J, Tursz T, Van Glabbeke M, van Pottelsberghe C: Phase II study with docetaxel (taxotere) in advanced soft tissue sarcomas of the adult. EORTC Soft Tissue and Bone Sarcoma Group. Ann Oncol 5: 539–542, 1994
Kostler WJ, Brodowicz T, Attems Y, Hejna M, Tomek S, Amann G, Fiebiger WC, Wiltschke CH, Krainer M, Zielinski CC: Docetaxel as rescue medication in anthracycline-and ifosfamide-resistant locally advanced or metastatic soft tissue sarcoma: results of a phase II trial. Ann Oncol 12: 1281–1288, 2001
Edmonson JH, Ebbert LP, Nascimento AG, Jung SH, McGaw H, Gerstner JB: Phase II study of docetaxel in advanced soft tissue sarcomas. Am J Clin Oncol 19: 574–576, 1996
Verweij J, Lee SM, Ruka W, Buesa J, Coleman R, van Hoessel R, Seynaeve C, di Paola ED, Van Glabbeke M, Tonelli D, Judson IR: Randomized phase II study of docetaxel versus doxorubicin in first-and second-line chemotherapy for locally advanced or metastatic soft tissue sarcomas in adults: a study of the european organization for research and treatment of cancer soft tissue and bone sarcoma group. J Clin Oncol 18: 2081–2086, 2000
Delaloge S, Yovine A, Taamma A, Riofrio M, Brain E, Raymond E, Cottu P, Goldwasser F, Jimeno J, Misset JL, Marty M, Cvitkovic E: Ecteinascidin-743: a marine-derived compound in advanced, pretreated sarcoma patients—preliminary evidence of activity. J Clin Oncol 19: 1248–1255, 2001
Demetri GD: ET-743: the US experience in sarcomas of soft tissues. Anticancer Drugs 13(suppl 1): S7–S9, 2002
Kollmannsberger C, Brugger W, Hartmann JT, Maurer F, Bohm P, Kanz L, Bokemeyer C: Phase II study of oral trofosfamide as palliative therapy in pretreated patients with metastatic soft-tissue sarcoma. Anticancer Drugs 10: 453–456, 1999
Blomqvist C, Wiklund T, Pajunen M, Virolainen M, Elomaa I: Oral trofosfamide: an active drug in the treatment of soft-tissue sarcoma. Cancer Chemother Pharmacol 36: 263–265, 1995
Budd GT, Rankin C, Hutchins LF, Wong L, Petruska PJ, Antman K: Phase II trial of topotecan by continuous infusion in patients with advanced soft tissue sarcomas, a SWOG study. Southwest Oncology Group. Invest New Drugs 20: 129–132, 2002
Raney RB, Anderson JR, Barr FG, Donaldson SS, Pappo AS, Qualman SJ, Wiener ES, Maurer HM, Crist WM: Rhabdomyosarcoma and undifferentiated sarcoma in the first two decades of life: a selective review of intergroup rhabdomyosarcoma study group experience and rationale for Intergroup Rhabdomyosarcoma Study V. J Pediatr Hematol Oncol 23: 215–220, 2001
Van Glabbeke M, Verweij J, Judson I, Nielsen OS: Progression-free rate as the principal end-point for phase II trials in soft-tissue sarcomas. Eur J Cancer 38: 543–549, 2002
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Reichardt, P., Oechsle, K., Pink, D. et al. An open label, non-comparative phase II study of topotecan as salvage treatment for patients with soft tissue sarcoma. Invest New Drugs 21, 481–486 (2003). https://doi.org/10.1023/A:1026263604863
Issue Date:
DOI: https://doi.org/10.1023/A:1026263604863