Abstract
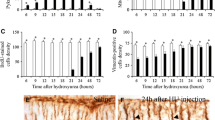
Purpose. Penclomedine (PEN), a multichlorinated α-picoline derivative which is metabolized to highly reactive alkylating species, was selected for clinical development due to its prominent activity against a wide range of human tumor xenografts when administered either parentally or orally. Its principal dose-limiting toxicity in preclinical and clinical studies has been neurocerebellar toxicity, which has been related to the magnitude of peak plasma PEN concentrations, but not to plasma concentrations of its putative principal alkylating metabolite, 4,o-demethylpenclomedine (DMPEN). These observation, as well as PEN's toxicologic, pharmacologic, and tissue distribution profiles, have suggested that the parent compound is primarily responsible for cerebellar toxicity. The studies described in this report were undertaken to characterize the neuropathology of PEN neurotoxicity, with a long-term goal of developing strategies to maximize its therapeutic index. Design. Male Sprague–Dawley rats were treated with therapeutically relevant doses of PEN, orally and intraperitoneally (i.p.), on various administration schedules, and DMPEN administered i.p. The animals were monitored for neurotoxicity, and brain sections were examined for neuropathology, particularly Purkinje cell loss and neuronal injury. Brain sections were stained using standard histochemical techniques and immunostained with OX-42 to detect microglial cells that are activated following neuronal damage, and calbindin D28K, a calcium-binding protein expressed by cerebellar Purkinje cells. Results. Dose-related neurocerebellar toxicity associated with parasagittal bands of Purkinje cell degeneration and microglial activation in the cerebellar vermis were evident in rats treated with PEN 100–400 mg/kg i.p. as a single dose. Neuronal injury was not observed in other regions of the brain. Furthermore, neither clinical nor histopathological evidence of cerebellar toxicity was apparent in rats treated with similar total doses of PEN administered i.p. on a daily×5-day dosing schedule. Similar histological findings, in an identical neuroanatomical distribution, were observed in rats treated with PEN orally; however, the magnitude of the neuronal toxicity was much less than in animals treated with equivalent doses of PEN administered i.p. Although acute lethality occurred in some rats treated with equimolar doses of DMPEN as a single i.p. treatment, surviving animals exhibited neither signs nor histopathological evidence of neurocerebellar toxicity. Conclusions. PEN produces selective dose- and schedule-dependent Purkinje cell degeneration in the cerebellar vermis of rats, whereas therapeutically relevant doses of PEN administered orally are better tolerated and produce less neurocerebellar toxicity. In addition, roughly equivalent, albeit intolerable, doses of the major active metabolite DMPEN, which was lethal to some animals, produced neither clinical manifestations of neurocerebellar toxicity nor Purkinje cell loss. These results support a rationale for investigating whether PEN administered orally, which may undergo significant first-pass metabolism to DMPEN and other less toxic intermediates, or treatment with DMPEN, itself, may result in less neurocerebellar toxicity and superior therapeutic indices than PEN administered parenterally.
Similar content being viewed by others
References
Plowman J, Harrison SD, Jr, Dykes DJ, Paull KD, Narayanan V, Tobol HK, Griswold DP, Jr: Preclinical antitumor activity of an alpha picoline derivative, penclomedine (NSC 338720), on human and murine tumors. Cancer Res 49: 1909–1915, 1989
Harrison SD, Jr, Plowman J, Dykes DJ, Waud WR and Griswold DP, Jr: Preclinical antitumor activity of penclomedine in mice: cross resistance, schedule dependence, and oral activity against tumor xenografts in brain. Cancer Res 51: 1979–1983, 1991
Reid JM, Mathieson DA, Benson LM, Kuffel MJ, Ames MM: Murine pharmacokinetics and metabolism of penclomedine [3,5–dichloro-2,4–dimethoxy-6–(trichloromethyl)pyridine, NSC 338720]. Cancer Res 52: 2830–2834, 1992
Hartman NR, O'Reilly S, Rowinsky EK, Collins J, Strong JM: Murine and human in vivo penclomedine metabolism. Clin Cancer Res 2: 953–962, 1996
O'Reilly S, Hartman NR, Grossman SA, Strong JM, Struck RF, Eller S, Lesser GJ, Donehower RC, Rowinsky EK: Tissue and tumor distribution of 14C-penclomedine in rats. Clin Cancer Res 2: 541–548, 1996
O'Reilly, S: Pharmacologic Studies of Penclomedine. Doctoral dissertation. Johns Hopkins University/Johns Hopkins University Press, Baltimore, MD, 1996
Waud WR, Tiwari A, Schmid SM, Shih TW, Strong JM, Hartman NR, O'Reilly S, Struck RF: 4–Demethylpenclomedine, an antitumor active, non-neurotoxic metabolite of penclomedine. Cancer Res 57: 815–817, 1997
Freidman H, Keir S, Bigner D, Struck, R: Treatment of CNS tumor xenografts with penclomedine and 4–demethylpenclomedine. Proc Amer Assoc Cancer Res 39: 218, 1998 (Abstract)
Division of Cancer Treatment, Diagnosis and Centers, National Cancer Institute: Penclomedine Investigators Brochure. National Cancer Institute, Bethesda, MD, 1992
O'Reilly S, Grochow LB, Donehower RC, Bowling K, Chen TL, Hartman N, Struck RF, Rowinsky EK: Phase I and pharmacologic study of penclomedine, a novel alkylating agent, in patients with solid tumors. J Clin Oncol 15: 1974–1984, 1997
Berlin J, Stewart JA, Storer B, Tutsch KD, Arzoomanian RZ, Alberti D, Feierabend C, Simon K, Wilding, G: Phase I clinical and pharmacokinetic trial of penclomedine using a novel, two-stage trial design for patients with advanced malignancy. J Clin Oncol 16: 1142–1149, 1998
Jodrell DI, Bowman A, Stewart M, Dunlop N, French R, MacLellan A, Cummings J, Smyth JF: Dose-limiting neurotoxicity in a phase I study of penclomedine (NSC 388720, CRC 88–04), a synthetic alpha-picoline derivative, administered intravenously. Br J Cancer 77: 808–811, 1998
Kreutzberg GW: Microglia: a sensor for pathological events in the CNS. Trends Neurosci 19: 312–318, 1996
Thomas WE: Brain macrophages: evaluation of microglia and their functions. Brain Res Rev 17: 61–74, 1992
Sequier JM, Hunziker W, Andressen C, Celio MR: Calbindin D28K protein and mRNA localization in the rat brain. Eur J Neurosci 2: 1118–1126, 1990
Freireich EJ, Gehan EA, Rall DP, Schmidt LH, Skipper HE: Quantitative comparison of toxicity of anti-cancer agents in mouse, rat, dog, monkey and man. Cancer Chemother Rep 50: 219–244, 1966
O'Hearn E, Long DB, Molliver ME: Ibogaine induces glial activation in parasagittal zones in the cerebellum. Neuro-Report 4: 299–302, 1993
Mamounas LA, Mullen CA, O'Hearn E, Molliver ME: Dual serotonergic projections to forebrain in the rat: morphologically distinct 5–HT axon terminals exhibit differential vulnerability to neurotoxic amphetamine derivatives. J Comp Neurol 314: 435–440, 1991
O'Hearn E, Molliver ME: Degeneration of Purkinje cells in parasagittal zones in the cerebellar vermis after treatment with ibogaine or harmaline. Neuroscience 55: 303–310, 1993
Näkki R, Koistinaho J, Sharp FR, Sagar SM: Cerebellar toxicity of phencyclidine. J Neurosci 15: 2097–2108, 1995
Llinás R, Yarom Y: Oscillatory properties of guinea-pig inferior olivary neurons and their pharmacological modulation: an in vitro study. J Physiol 376: 163–182, 1986
O'Hearn E, Molliver ME: The olivocerebellar projection mediates ibogaine-induced degeneration of Purkinje cells: a model of indirect, trans-synaptic excitotoxicity. J Neurosci 17: 8828–8841, 1997
O'Hearn E, Molliver ME: Neurotoxins and neuronal death: an animal model of excitotoxicity. In: Koliatsos VE, Ratan RR (eds) Cell Death and Diseases of the Nervous System. Human Press Inc., Totowa, NJ, 1999, pp 221–245
Nicotera P, Bellomo G, Orrenius S: Calcium-mediated mechanisms in chemically induced cell death. Annu Rev Pharmacol Toxicol 32: 449–470, 1992
Siman R, Noszek JC: Excitatory amino acids activate calpain I and induce structural protein breakdown in vivo. Neuron 1: 279–287, 1988
Llinás R, Sasaki K: The functional organization of the olivocerebellar system as examined by multiple Purkinje cell recordings. Eur J Neurosci 1: 587–602, 1989
Llinás R, Nicholson C: Reversal properties of climbing fiber potential in cat Purkinje cells: an example of a distributed synapse. J Neurophysiol 39: 311–323, 1976
Winkelman MD, Hines JD: Cerebellar degeneration caused by high dose cytosine arabinoside: a clinicopathologic study. Ann Neurol 14: 520–527, 1983
Wetts R, Moran T, Oster-Granite M, Gearhart J: Effect of Purkinje cell loss on complex motor behavior. Soc Neurosci 11: 1037, 1985 (Abstract)
O'Reilly S, Strong J, Bowling K, Rowinsky EK, Donehower RC, Collins J, Hartman NR: A pharmacologic study of the oral bioavailability study of oral penclomedine. Cancer Chemother Pharmacol 48: 223–228, 2001
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
O'Reilly, S., O'Hearn, E., Struck, R.F. et al. The alkylating agent penclomedine induces degeneration of purkinje cells in the rat cerebellum. Invest New Drugs 21, 269–279 (2003). https://doi.org/10.1023/A:1025456224751
Issue Date:
DOI: https://doi.org/10.1023/A:1025456224751