Abstract
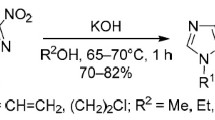
The treatment of 5-hydrazono-1,2,3-thiadiazoles by phosphorus pentachloride in toluene or xylene leads to an anomalous Dimroth rearrangement and the reaction of the mercapto function formed with the methyl group of the solvent to give 5-benzylmercapto-1,2,3-triazole.
Similar content being viewed by others
REFERENCES
T. Kindt-Larsen andC. Pedersen, Acta Chem. Scand., 16, 1800 (1962).
W. Dehaen,M. Voets, andV. A. Bakulev, in: Advances in Nitrogen Heterocycles, JAI Press, Inc., Vol. 4, Stamford, Connecticut (2000), p. 37.
Yu. M. Shafran,V. A. Bakulev,V. A. Shevyrin, andM. Yu. Kolobov, Khim. Geterotsikl. Soedin., 840 (1993).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Glukhareva, T.V., Dyudya, L.V., Morzherin, Y.Y. et al. Reaction of 5-Hydrazono-1,2,3-thiadiazoles with Toluene and Xylene in the Presence of PCl5 . Chemistry of Heterocyclic Compounds 39, 126–127 (2003). https://doi.org/10.1023/A:1023041329732
Issue Date:
DOI: https://doi.org/10.1023/A:1023041329732