Abstract
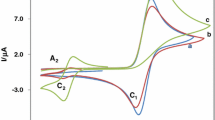
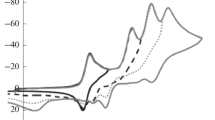
Classical polarography, cyclic voltammetry, and EPR spectroscopy was used to study electrochemical reduction and oxidation of 3-nitro derivatives of 2-methyl-4-phenylquinoline, the corresponding quinolinium perchlorates, and 1,2- and 1,4-dihydroquinolines. The nitro derivatives of quinoline and 1,2-dihydroquinoline are reduced in the first step at the nitro group; the quinolinium cations are reduced at the heterocycle followed by reduction of the nitro group; and in 1,4-dihydroquinolines, the nitro group is not reduced. Electrochemical reduction processes associated with electron transfer in the heterocycle mainly display the same behavior as established for pyridine derivatives. But important differences were observed in electrochemical oxidation: the N-methyl derivative of 1,4-dihydroquinoline is oxidized significantly more easily than the corresponding N-unsubstituted derivative of 1,4-dihydroquinoline (in the 1,4-dihydropyridine series, the difference in pot! enti als is fairly small), and even more easily than the corresponding N-methyl derivative of 1,2-dihydroquinoline.
Similar content being viewed by others
REFERENCES
Y. Kikugawa, H. Kuramoto, I. Saito, and S. Jamada, Chem. Pharm. Bull., 21, 1914 (1973).
R. M. I. Roberts, D. Ostovic, and M. M. Kreevoy, J. Org. Chem., 48, 2053 (1983).
G. A. Dauphinee and T. P. Forrest, Canad. J. Chem., 56, 632 (1978).
B. A. Vigante, Ya. Ya. Ozols, and G. Ya. Dubur, Khim. Geterotsikl. Soedin., 1680 (1991).
B. A. Vigante, Ya. Ya. Ozols, and G. Ya. Dubur, Khim. Geterotsikl. Soedin., 1696 (1989). 222
D. J. Casimir and L. E. Lyons, J. Chem. Soc., 783 (1950).
A. G. Stromberg and T. M. Markacheva, Zh. Fiz. Khim., 57 (1954).
M. Maturova, V. Preininger, and F. Santavy, Abhandl. Deutsch. Akad. Wiss. Berlin, Kl. Chem. Geol. Biol., 1, 85 (1964).
T. Fujinaga, K. Izutsu, and K. Takaoka, J. Electroanal. Chem., 12, 203 (1966).
T. A. Mikhailova, N. I. Kudryashova, and N. V. Khromov-Borisov, Zh. Obshch. Khim., 39, 26 (1969).
J. Stradins, R. Gavars, and L. Baumane, Elektrokhimiya, 31, 1100 (1995).
W. Kemula and Z. Kublik, Roczn. Chem., 32, 941 (1958).
L. Baumane, R. Gavar, J. Stradins, and G. Dubur, Khim. Geterotsikl. Soedin., 219 (1997).
L. Lunazzi, A. Mangini, G. F. Pedulli, and F. Taddei, J. Chem. Soc. B., 163 (1970).
J. Stradins, R. Gavars, L. Baumane, B. Vigante, and G. Duburs, Khim. Geterotsikl. Soedin., 1222 (1996).
J. Stradins, R. Gavars, L. Baumane, B. Vigante, and G. Duburs, Khim. Geterotsikl. Soedin., 212 (1997).
S. Kubota and Y. Ikegami, J. Phys. Chem., 82, 2739 (1978).
R. A. Gavar, L. Kh. Baumane, Ya. P. Stradyn', V. K. Lusis, G. Ya. Dubur, and D. Kh. Mutsenietse, Khim. Geterotsikl. Soedin., 708 (1986).
P. J. Elving and M. S. Spritzer, Talanta, 12, 1243 (1965).
J. Hermolin, M. Levin, Y. Ikegami, M. Sawayanagi, and E. M. Kosower, J. Am. Chem. Soc., 103, 4795 (1981).
K. Akiyama, S. Tero-Kubota, and Y. Ikegami, J. Am. Chem. Soc., 105, 3601 (1983).
J. Stradins, R. Gavars, L. Baumane, B. Vigante, and G. Duburs, Khim. Geterotsikl. Soedin., 355 (1995).
Ya. Ozols, B. Vigante, L. Baumane, A. Mishnev, I. Turovskis, G. Duburs, and J. Stradins, Khim. Geterotsikl. Soedin., 1372 (1998).
J. Ogle, J. Stradins, and L. Baumane, Electrochem. Acta, 39, 73 (1993).
J. N. Burnett and A. L. Underwood, Biochemistry, 30, 1154 (1965).
R. D. Clark and C. H. Heathcock, J. Org. Chem., 41, 636 (1976).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Stradins, J., Baumane, L., Vigante, B. et al. Electrochemical Conversions of 2-Methyl-3-nitro-4-phenylquinoline, Its Quinolinium Salts, and Hydrogenated Derivatives. Chemistry of Heterocyclic Compounds 37, 212–223 (2001). https://doi.org/10.1023/A:1017519600449
Issue Date:
DOI: https://doi.org/10.1023/A:1017519600449