Abstract
Background: Chemotherapy remains theprimary mode of treatment for metastaticcarcinoma of the esophagus. The efficacyof various chemotherapeutic regimens hasbeen studied predominantly in patients withsquamous cell carcinoma of the esophagus. In light of the increasing incidence ofadenocarcinoma of the esophagus, studiesevaluating newer chemotherapy agents, suchas docetaxel, in this patient populationare necessary. The objective of this trialwas to determine the complete and partialresponse rate of docetaxel in patients withincurable adenocarcinoma of theesophagus.
Patients and methods: Eligiblepatients had histologically confirmedmetastatic adenocarcinoma of the esophagusor locally extensive disease not curablewith surgery or radiation therapy. Patients were either chemotherapy naive orpreviously treated with chemotherapy(including paclitaxel). Docetaxel wasadministered at a dose of 75 mg/m2every three weeks intravenously.Appropriate imaging studies/examinationswere obtained after every two cycles toevaluate response.
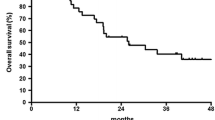
Results: A total of 22 patients wereenrolled in the trial. Chemotherapy-naivepatients achieved a response rate of 18%(95% CI = 2.3 to 51.8) while patients whoreceived prior chemotherapy achieved a 0%response rate (95% CI = 0 to 25). Therewere no complete responses. The overallmedian survival time is 3.4 months and theone-year survival rate is 21%. Thetoxicities included febrile neutropenia(32%) as well as grade 3 and 4 fatigue(14%) and anorexia (9%).
Conclusions: Although chemotherapy naivepatients achieved an 18% response rate andno responses were seen in previouslytreated patients, the limitations of thistrial does not allow for any definitiveconclusions to be made about the efficacyof single agent docetaxel chemotherapy inpatients with incurable esophagealcancer.
Similar content being viewed by others
References
Blot WJ, Devesa SS, Kneller RW, Fraumeni JF Jr: Rising incidence of adenocarcinoma of the esophagus and gastric cardia. JAMA 265: 1287–1289, 1991
Ajani JA: Contributions of chemotherapy in the treatment of carcinoma of the esophagus: results and commentary. Semin Oncol 21: 474, 1994
Engstrom PF, Lavin PT, Klaassen DJ: Phase II evaluation of mitomycin and cisplatin in advanced esophageal carcinoma. Cancer Treat Rep 67: 713, 1983
Coonley CJ, Bains M, Heelan R, Dukeman M, Kelsen DP: Phase II study of etoposide in the treatment of esophageal carcinoma. Cancer Treat Rep 67: 397, 1983
Bissery MC, Nohynek G, Sanderink GJ, Lavelle F: Docetaxel: A review of preclinical and clinical experience – Part I. Preclinical experience. Anticancer Drugs 6: 339–368, 1995
Riou JF, Naudin A, Lavelle F: Effects of Taxotere on murine and human tumor cell lines. Biochem Biophys Res Commun 187: 164–170, 1992
Tanaka M, Obata T, Sasaki T: Evaluation of antitumour effects of docetaxel (Taxotere) on human gastric cancers in vitro and in vivo. Eur J Cancer 32A: 226–30, 1996
Ajani JA, Ilson DH, Daugherty K, Pazdur R, Lynch PM, Kelsen DP: Activity in patients with squamous cell carcinoma and adenocarcinoma of the esophagus. J Natl Cancer Inst 86: 1086, 1994
Einzig AI, Neuberg D, Remick SC, Karp DD, O'Dwyer PJ, Stewart JA, Benson AB: Phase II trial of docetaxel (Taxotere) in patients with adenocarcinoma of the upper gastrointestinal tract previously untreated with cytotoxic chemotherapy: the Eastern Cooperative Oncology Group (ECOG) results of the protocol E1293. Med Oncol 13: 87–93, 1996
Simon R: Optimal two-stage designs for phase II clinical trials. Control Clin Trials 10: 1–10, 1989
Fossella FV, DeVore R, Kerr R, Crawford J, Natale RR, Dunphy F, Kalman L, Miller V, Lee JS, Moore M, Gandara D, Karp D, Vokes E, Kris M, Kim Y, Gamza F, Hammershaimb L: Phase III trial of docetaxel 100 mg/m2 or 75 mg/m2 vs vinorelbine/ifosfamine for non-small cell lung cancer (NSCLC) previously treated with platinum-based chemotherapy (PBC). Am Soc Clin Oncol 1776, 1999
Kavanagh JJ, Winn R, Steger M, Nelson-Taylor T, Edwards K, Rodgers R, Borst J, Kudelka A, Hu W, Verschraegen CF: Docetaxel for patients with ovarian cancer refractory to paclitaxel, an update. Am Soc Clin Oncol 1423, 1999
Valero V, Jones SE, Von Hoff DD, Booser DJ, Mennel RG, Ravdin PM, Holmes FA, Rahman Z, Schottstaedt MW, Erban JK, Esparza-Guerra L, Earhart RH, Hortobagyi GN, Burris HA: A phase II study of docetaxel in patients with paclitaxel-resistant metastatic breast cancer. J Clin Oncol 16: 3362–3368, 1998
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Heath, E.I., Urba, S., Marshall, J. et al. Phase II Trial of Docetaxel Chemotherapy in Patients with Incurable Adenocarcinoma of the Esophagus. Invest New Drugs 20, 95–99 (2002). https://doi.org/10.1023/A:1014476602804
Issue Date:
DOI: https://doi.org/10.1023/A:1014476602804