Abstract
Cantharidin (Spanish Fly) is a naturaltoxin and an inhibitor of proteinphosphatases 1 (PP1) and 2A (PP2A), whichhave key roles in cell cycle progression.We have synthesised two series ofdemethylated cantharidin analogues, onedisplaying an open-ring lactoneconfiguration in solution (Novo-1 toNovo-5) similar to cantharidin, the othershowing a closed-ring lactone configuration(Novo-6 to Novo-10). In the present study,these ten agents were screened for invitro PP1 and PP2A inhibition and cellularcytotoxicity in nine cancer cell lines ofhaematopoietic (L1210, HL60), ovarian(A2780, ADDP), osteo (143B), and colon(HCT116, HT29, WiDr, SW480) origin and onenormal colon cell line (CCD-018).
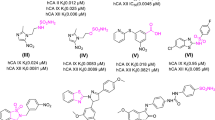
The open-ring series (IC50,PP1=2.0−4.8 μM, PP2A=0.2−0.5 μM)maintained the PP2A selectivity ofcantharidin (IC50, PP1=1.8 μM,PP2A=0.2 μM), although some were lesspotent. The closed-ring series (IC50,PP1 = 12.5->1000 μM,PP2A=5->1000 μM) were considerablyless potent inhibitors, confirming the needof ring opening for inhibition. Thecytotoxicity (IC50, 72 h, MTT assay) ofcantharidin ranged from 6−15 μM, whilethe new analogues ranged from 14 to>1000 μM. Cytotoxicity of the agentsdid not consistently parallel the invitro potency of protein phosphataseinhibition. A number of analogues showedcolon cancer selectivity, particularlyNovo-6, where the cytotoxicity ranged from14−88 μM in the colon cancer cells and275−680 μM in all other cell linesincluding normal colon cells. The reasonfor this selectivity was not apparent andmay involve additional intracellulartargets. Cell cycle analysis showedcantharidin to enhance cell cycleprogression as evident from an increasedS-phase population and enhanced DNAsynthesis, culminating in G2/M arrestand apoptosis. With Novo-1 and Novo-6, thecell cycle changes paralleled thecytotoxicity responses, with thepredominant effect of G2/M cell cyclearrest followed by cell death. Inconclusion, we have synthesised newanticancer agents that show selectivecytotoxicity in colon cancer cells whileremaining inactive in normal colon cells,and which mediate their effects viathe G2/M phase of the cell cycle.
Similar content being viewed by others
References
Southcott CV: Injuries from Coleoptera. Med J Aust 151: 654–659, 1989
Nicholls LC, Teare D: Poisoning by cantharides. Br J Med 2: 1384–1386, 1954
Rosin RD: Cantharides intoxication. Br Med J 4: 33–33, 1967
Polettini A, Crippa O, Ravagli A, Saragoni A: A fatal case of poisoning with cantharidin. Forensic Science Int 56: 37–43, 1992
Karras DJ, Farrell SE, Harrigan RA, Henretig FM, Gealt L: Poisoning from “Spanish Fly”. Am J Emergency Medicine 14: 478–483, 1996
Graziano MJ, Waterhouse AL, Casida JE: Cantharidin poisoning associated with specific binding site in liver. Biochem Biophys Res Commun 149: 79–85, 1987
Wang G-S: Medical uses of mylabris in ancient China and recent studies. J Ethnopharmacol 26: 147–162, 1989
Liu X-H, Balzsek I, Comisso M, Legras S, Marion S, Quittet P, Anjo A, Wang G-S: Effects of norcantharidin, a protein phosphatase type-2A inhibitor, on the growth of normal and malignant haemopoietic cells. Eur J Cancer 31A: 953–963, 1995
Li Y-M, Casida JE: Cantharidin-binding protein: Identification as protein phosphatase 2A. Proc Natl Acad Sci USA 89: 11867–11870, 1992
de Jong RS, de Vries EGE, Meijer S, de Jong PE, Mulder NH: Renal toxicity of the anticancer drug fostriecin. Cancer Chem Pharm 42: 160–164, 1998
de Jong RS, Mulder NH, Uges DRA, Sleijfer DT, Hoppener FJP, Groen HJM, Willemse PHB, van der Graaf WTA, de Vries EGE: Phase 1 and pharmacokinetic study of the topoisomerase II catalytic inhibitor. Br J Cancer 79: 882–887, 1999
Wera S, Hemmings BA: Serine/threonine protein phosphatases. Biochem J 311: 17–29, 1995
Morana SJ, Wolf CM, Li JF, Reynolds JE, Brown MK, Eastman A: The involvement of protein phosphatases in the activation of ICE/CED-3 protease, intracellular acidification, DNA digestion, and apoptosis. J Biol Chem 271: 18263–18271, 1996
Wolf CM, Morana SJ, Eastman A: Zinc inhibits apoptosis upstream of ICE/CED-9 proteases rather than at the level of an endonuclease. Cell Death Differ 4: 125–129, 1997
Puri PL, MacLachlan TK, Levrero M, Giordano A: The intrinsic cell cycle: From yeast to mammals. In: Stein GS, Baserga R, Giordano A, Denhardt DT (eds) The molecular basis of cell cycle growth control. Wiley-Liss, New York, 1999, pp 15–79
Draetta G, Eckstein J: Cdc25 protein phosphatases in cell proliferation. Genes Dev 1332: M53-M63, 1997
O'Connor PM: Mammalian G1 and G2 phase checkpoints. Cancer Surveys 29: 151–182, 1997
Pines J: Cyclins and cyclin-dependent kinases: Theme and variations. Adv Cancer Res 66: 181–212, 1995
Millward TA, Zolnierowicz S, Hemmings BA: Regulation of protein kinase cascades by protein phosphatase 2A. TIBS 24: 186–191, 1999
Roberge M, Tudan C, Hung SM, Harder KW, Jirik FR: Antitumor drug fostriecin inhibits the mitotic entry checkpoint and protein phosphatases 1 and 2A. Cancer Res 54: 6115–6121, 1994
Yamashita K, Yasuda H, Pines J, Yasumoto K, Nishitani H, Ohtsubo M, Hunter T, Sugimura T, Nishimoto T: Okadaic acid, a potent inhibitor of type 1 and 2A protein phosphatases, activates cdc2/H1 kinase and transiently induces a premature mitosis-like state in BHK21 cells. EMBO J 9: 4331–4338, 1990
McCluskey A, Taylor C, Quinn RJ, Suganuma M, Fujiki H: Inhibition of protein phosphatase 2A by cantharidin analogues. BioMed Chem Let 6: 1025–1028, 1996
McCluskey A, Bowyer MC, Collins E, Sim ATR, Sakoff JA, Baldwin ML: Anhydride modified Cantharidin analogues: Synthesis, anti-cancer activity and selective inhibition of protein phosphatase 1 and 2A. Bioorg Med Chem Let 10: 1687–1690, 2000
McCluskey A, Keane MA, Mudgee L-M, Sim ATR, Sakoff JA, Quinn RJ: Anhydride modified cantharidin analogues. Is ring opening important in the inhibition of protein phosphatase 2A? Eur J Med Chem 35: 957–964, 2000
Sakoff JA, Ackland SP: Thymidylate synthase inhibition induces S-phase arrest, biphasic mitochondrial alterations and caspase dependent apoptosis in leukaemia cells. Cancer Chem Pharm 46: 477–487, 2000
Bergman AM, Ruiz van Haperen VW, Veerman G, Kuiper CM, Peters GJ: Synergistic interaction between cisplatin and gemcitabine in vitro. Clin Cancer Res 2: 521–530, 1996
Rajesh D, Stenzel RA, Verma AK: Protein phosphatases and apoptosis: Protein phosphatases 1 and 2A, as potential components of cell death pathways in human cancer cell lines. Proc Am Ass Cancer Res 40: 222, 1999
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sakoff, J.A., Ackland, S.P., Baldwin, M.L. et al. Anticancer Activity and Protein Phosphatase 1 and 2A Inhibition of a New Generation of Cantharidin Analogues. Invest New Drugs 20, 1–11 (2002). https://doi.org/10.1023/A:1014460818734
Issue Date:
DOI: https://doi.org/10.1023/A:1014460818734