Abstract
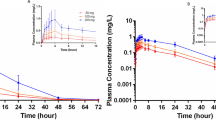
The pharmacokinetics, efficacy and safety of metoprolol tartrate 25 mg fatty suppositories were studied in 5 healthy volunteers and in 8 patients suffering from instable angina pectoris. Metoprolol 25 mg capsules were used as a control oral dosage form. Metoprolol showed a considerable rectal bioavailability (AUC, C max) and was absorbed quickly from the rectum (T max). In both groups rectal bioavailability was comparable. However, oral bioavailability was much lower in the volunteer group than in the patient group. Furthermore, ratios of metoprolol/a‐OH‐metoprolol concentrations in plasma and urine gave an indication for a partial avoidance of the first pass effect after rectal administration. Further research is necessary to define an exact rectal dosage of metoprolol. In all patients, a substantial drop in heart rate, systolic and diastolic blood pressure was seen after administration of the first suppository. Metoprolol suppositories appear to be an effective, safe and suitable alternative for patients who are in need for beta blocking medication and who are unable to take oral medication for a certain amount of time.
Similar content being viewed by others
References
Van Hoogdalem EJ, de Boer AG, Breimer DD. Pharmacokinetics of rectal drug administration, part I. Clin Pharmacokinet 1991;21;11-26
Van Hoogdalem EJ, de Boer AG, Breimer DD. Pharmacokinetics of rectal drug administration, part II. Clin Pharmacokinet 1991;21;110-28
Plosker GL, Glissold SP. Controlled release metoprolol formulations: a review of their pharmacodynamic and pharmacokinetic properties and therapeutic use in hypertension and ischaemic heart disease. Drugs 1992;43:382-414
McCourty JC, Silas JH, Lennard MS, Tucker GT, Woods HF. Metoprolol metabolism and debrisoquine oxidation polymorphism. Population and family studies. Br J Clin Pharmacol 1985;20(6):555-66
De Boer AG. First pass elimination of some high clearance drugs following rectal administration to humans and rats. Ph D thesis, University of Leiden, 1979
Cid E, Mella F, Lucchini L, Carcamo M, Monasterio J. Plasma concentrations and bio-availability of propranolol by oral, rectal and intravenous administration in man. Biopharm Drug Disp 1986;7:559-66
Groothuysen H, Hoogtanders K, de Stoppelaar F, Stolk L. Determination of metoprolol and alfa-hydroxymetoprolol in plasma. Ziekenhuisfarmacie 1998;14:237-8
Proost JH, Meijer DKF. MW/PHARM, an integrated software package for drug dosage regimen calculation and therapeutic drug monitoring. Comput Biol Med 1992;22:155-63
Lennard MS, Silas JH, Freestone S Trevethick J. Defective metabolism of metoprolol in poor hydroxylators of debrisoquine. Br J Clin Pharmacol 1982;14:301-3
Lennard MS, Silas JH, Lawrence ER, Tucker GT, Woods HF. Oxidation phenotype-a major determinant of metoprolol metabolism and response. New Engl J Med 1982;307(25):1558-60
Laurent-Kenesi MA, Funck-Brentano C, Poirier JM, Decolin D, Jaillon P. Influence of CYP2D6-dependent metabolism on the steady state pharmacokinetics and pharmacodynamics of metoprolol and nicardipine, alone and in combination. Br J Clin Pharmacol 1993;36:531-8
Otton SV, Crewe HK, Lennard MS, Tucker GT, Woods HF. Use of quinidine inhibition to define the role of the sparteine/ debrisoquine cytochrome P450 in metoprolol oxidation by human liver microsomes. J Pharmacol Exp Ther 1988;247(1):242-7
Jonkers RE, Koopmans RP, Portier EJG, van Boxtel CJ. Debrisoquine phenotype and the pharmacokinetics and beta-2 receptor pharmacodynamics of metoprolol and its enantiomers. J Pharmacol Exp Ther 1991;256(3):959-66
Everts B, Karlson BW, Herlitz J, Abdon NJ, Hedner T. Effects and pharmacokinetics of high dose metoprolol on chest pain in patients with suspected or definite acute myocardial infarction. Eur J Clin Pharmacol 1997;53:23-31
Regardh CG, Jordö L, Ervik M, Lundborg P, Olsson R, Rönn O. Pharmacokinetics of metoprolol in patients with hepatic cirrhosis. Clin Pharmacokin 1981;6:375-88
Regardh CG, Landahl S, Larsson M, Lundborg P, Steen B, Hoffmann KJ, Lagerström PO. Pharmacokinetics of metoprolol and its metabolite α-OH-metoprolol in healthy, nonsmoking, elderly individuals. Eur J Clin Parmacol 1983;24:221-6
Sanberg A, Abrahamsson B, Regardh CG, Wieselgren I, Bergstrand R. Pharmacokinetic and biopharmaceutic aspects of once daily treatment with metoprolol CR/ZOK: A review article. J Clin Pharmacol 1990;30:S2-S16
Rights and permissions
About this article
Cite this article
de Stoppelaar, F., Stolk, L., Beysens, A. et al. The relative bioavailability of metoprolol following oral and rectal administration to volunteers and patients. Pharm World Sci 21, 233–238 (1999). https://doi.org/10.1023/A:1008792421982
Issue Date:
DOI: https://doi.org/10.1023/A:1008792421982