Abstract
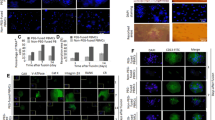
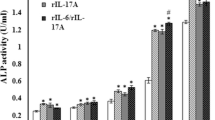
The osteoclasts are bone-resorbing multinucleatedcells formed by the fusion of mononuclearpreosteoclasts (pOCs) of hematopoietic origin.Although receptor activator of NF-κBligand (RANKL) has been shown to regulate osteoclastdifferentiation and function, its effect on the fusionof pOCs into multinucleated osteoclast-like cells(OCLs) has not been known. Using our fusion assaysystem, that is not contaminated with multinucleatedcells (MNCs) and osteoblastic cells, we determined theeffect of RANKL on the fusion of pOCs into MNCs. WhenpOCs were cultured on the plates, most of pOCs diedand disappeared from the plates within 24 h in theabsence of additives, but pOCs were fused to MNCswithin 6 h in the presence of RANKL. RANKL-inducedMNCs showed typical properties of OCL such astartrate-resistant acid phosphatase (TRAP) activity,actin ring formation, and bone-resorbing activity. Thefusion of pOCs into OCLs induced by osteoblastic cellsor RANKL was inhibited by OPG/OCIF, but that inducedby IL-1β was not. Both RANKL- andIL-1β-induced OCL formation from pOCs wasinhibited by ZLLL-H, a peptide inhibitor ofproteasome. These findings indicate that RANKLsupports the survival of pOCs and induces the fusionof pOCs into OCLs and suggest that NF-κBactivation is involved in these processes induced byRANKL and IL-1β.
Similar content being viewed by others
References
Akatsu T, Murakami T, Nishikawa M, Ono K, Shinomiya N, Tsuda E, Mochizuki SI, Yamaguchi K, Kinosaki M, Higashio K, Yamamoto M, Motoyoshi K and Nagata N (1998) Osteoclastogenesis inhibitory factor suppresses osteoclast survival by interfering in the interaction of stromal cells with osteoclasts. Biochem Biophy Res Commun 250: 229–234.
Anderson DM, Maraskovsky E, Billingsley WL, Dougall WC, Tometsko ME, Roux ER, Teepe MC, DuBose RF, Cosman D and Galibert L (1997) A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature 390: 175–179.
Athanasou NA (1996) Cellular biology of bone-resorbing cells. J Bone Joint Surg Am 78: 1096–1112.
Baeuerle PA and Baltimore D (1996) NF-κB: Ten years after. Cell 87: 13–20.
Baldwin AS (1996) The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol 14: 649–683.
Darnay BG, Haridas V, Ni J, Moore PA and Aggarwal BB (1998) Characterization of the intracellular domain of receptor activator of NF-κB (RANK). Interaction with tumor necrosis factor receptor-associated factors and activation of NF-κB and c-Jun N-terminal kinase. J Biol Chem 273: 20551–20555.
Fuller K, Wong B, Fox S, Choi Y and Chambers TJ (1998) TRANCE is necessary and sufficient for osteoblast-mediated activation of bone resorption in osteoclasts. J Exp Med 188: 997–1001.
Jimi E, Shuto T and Koga T (1995) Macrophage colony-stimulating factor and interleukin-1 alpha maintain the survival of osteoclast-like cells. Endocrinology 136: 808–811.
Jimi E, Nakamura I, Ikebe T, Akiyama S, Takahashi N and Suda T (1998) Activation of NF-κB is involved in the survival of osteoclasts promoted by interleukin-1. J Biol Chem 273: 8799–8805.
Jimi E, Nakamura I, Duong LT, Ikebe T, Takahashi N, Rodan GA and Suda T (1999) Interleukin 1 induces multinucleation and bone-resorbing activity of osteoclasts in the absence of osteoblasts/stromal cells. Exp Cell Res 247: 84–93.
Hsu H, Lacey DL, Dunstan CR, Solovyev I, Colombero A, Timms E, Tan HL, Elliott G, Kelley MJ, Sarosi I, Wang L, Xia XZ, Elliott R, Chiu L, Black T, Scully S, Capparelli C, Morony S, Shimamoto G, Bass MB and Boyle WJ (1999) Tumor necrosis factor receptor family member RANK mediates osteoclast differentiation and activation induced by osteoprotegerin ligand. Proc Natl Acad Sci USA 96: 3540–3545.
Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian YX, Kaufman S, Sarosi I, Shalhoub V, Senaldi G, Guo J, Delaney J and Boyle WJ (1998) Osteprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93: 165–176.
Malinin NL, Boldin MP, Kovalenko AV and Wallach D (1997) MAP3K-related kinase involved in NF-κB induction by TNF, CD95 and IL-1. Nature 385: 540–544.
Mercurio F, Zhu H, Murray BW, Shevchenko A, Bennett BL, Li J, Young DB, Barbosa M, Mann M, Manning A and Rao A (1997) IKK-1 and IKK-2: cytokine-activated IκB kinases essential for NF-κB activation. Science 278: 860–866.
Nakagawa N, Kinosaki M, Yamaguchi K, Shima N, Yasuda H, Yano K, Morinaga T and Higashio K (1998) RANK is the essential signaling receptor for osteoclast differentiation factor in osteoclastogenesis. Biochem Biophys Res Commun 253: 395–400.
Nakamura I, Takahashi N, Sasaki T, Tanaka S, Udagawa N, Murakami H, Kimura K, Kabuyama Y, Kurokawa T, Suda T and Fukui Y (1995) Wortmannin, a specific inhibitor of phosphatidylinositol-3 kinase, blocks osteoclastic bone resorption. FEBS Lett 361: 79–84.
Regnier CH, Song HY, Gao X, Goeddel DV, Cao Z and Rothe M (1997) Identification and characterization of an IκB kinase. Cell 90: 373–383.
Rothe M, Wong SC, Henzel WJ and Goeddel DV (1994) A novel family of putative signal transducers associated with the cytoplasmic domain of the 75 kDa tumor necrosis factor receptor. Cell 78: 681–692.
Roodman GD (1996) Advances in bone biology: the osteoclasts. Endocrine Rev 17: 308–332.
Scheven BA, Visser JW and Nijweide PJ (1986) In vitro osteoclast generation from different bone marrow fractions, including a highly enriched haematopoietic stem cell population. Nature 321: 79–81.
Siebenlist U, Franzoso G and Brown K (1994) Structure, regulation and function of NF-κB. Annu Rev Cell Biol 10: 405–455.
Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Luthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, Shimamoto G, DeRose M, Elliott R, Colombero A, Tan HL, Trail G, Sullivan J, Davy E, Bucay N, Renshaw-Gegg L, Hughes TM, Hill D, Pattison W, Campbell P and Boyle WJ (1997) Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell 89: 309–319.
Song HY, Regnier CH, Kirschning CJ, Goeddel DV and Rothe M (1997) Tumor necrosis factor (TNF)-mediated kinase cascades: bifurcation of nuclear factor-κB and c-jun N-terminal kinase (JNK/SAPK) pathways at TNF receptor-associated factor 2. Proc Natl Acad Sci USA 94: 9792–9796.
Suda T, Nakamura I, Jimi E and Takahashi N (1997) Regulation of osteoclast function. J Bone Miner Res 12: 869–879.
Suda T, Udagawa N and Takahashi N (1996) Cells of bone: osteoclast generation. In: Bilezikian JP, Rase LG and Rodan GA (eds) Principles of bone biology (pp. 87–102). Academic Press San Diego.
Suda T, Takahashi N and Martin TJ (1995) Modulation of osteoclast differentiation: Update. Endocrine Rev 4: 266–270.
Takami M, Woo JT and Nagai K (1998) Requirement of osteoblastic cells for the fusion of preosteoclasts. J Bone Miner Metabol 16: 151–157.
Takahashi N, Akatsu T, Udagawa N, Sasaki T, Yamaguchi A, Moseley JM, Martin, TJ and Suda T (1988) Osteoblastic cells are involved in osteoclast formation. Endocrinology 123: 2600–2602.
Wesolowski G, Duong LT, Lakkakorpi PT, Nagy RM, Tezuka K, Tanaka H, Rodan GA and Rodan SB (1995) Isolation and characterization of highly enriched, prefusion mouse osteoclastic cells. Exp Cell Res 219: 679–686.
Wong BR, Rho J, Arron J, Robinson E, Orlinick J, Chao M, Kalachikov S, Cayani E, Bartlett FS, Frankel WN, Lee SY and Choi Y (1997a) TRANCE is a novel ligand of the tumor necrosis factor receptor family that activates c-Jun N-terminal kinase in T cells. J Biol Chem 272: 25190–25194.
Wong BR, Josien R, Lee SY, Sauter B, Li HL, Steinman RM and Choi Y (1997b) TRANCE (tumor necrosis factor [TNF]-related activation-induced cytokine), a new TNF family member predominantly expressed in T cells, is a dendritic cell-specific survival factor. J Exp Med 186: 2075–2080.
Wong BR, Josien R, Lee SY, Vologodskaia M, Steinman RM and Choi Y (1998) The TRAF family of signal transducers mediates NF-κB activation by the TRANCE receptor. J Biol Chem 273: 28355–28359.
Woronicz JD, Gao X, Cao Z, Rothe M and Goeddel DV (1997) IκB kinase-beta: NF-κB activation and complex formation with IκB kinase-alpha and NIK. Science 278: 866-869.
Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A, Tsuda E, Morinaga T, Higashio K, Udagawa N, Takahashi N and Suda T (1998) Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci USA 95: 3597–3602.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Woo, JT., Kato, M., Takami, M. et al. Receptor activator of NF-κB ligand induces the fusion of mononuclear preosteoclasts into multinucleated osteoclasts. Cytotechnology 33, 203–211 (2000). https://doi.org/10.1023/A:1008198120670
Issue Date:
DOI: https://doi.org/10.1023/A:1008198120670