Abstract
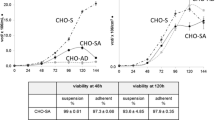
The murine hybridoma (CC9C10) was subjected to high shear rates in a spinner flask to determine the effect of various culture additives on cell survival. At 500 rpm, the half-life of the viable cell concentration in a low protein serum-free medium was 50 min. Both bovine serum albumin and Pluronic F-68 had a significant effect in protecting cells under these conditions. The effects of the two supplements were additive, so that in the presence of both supplements there was minimal cell damage at 500 rpm. The survival rate of cells grown in media supplemented with linoleic acid improved significantly under high stirring rates. Cells grown for one passage in 50 μM linoleic acid and stirred at 500 rpm had a significantly higher survival rate than control cells. For cells grown over 5 passages in 25 μM linoleic acid, the survival rate at 470 rpm was ×3 greater than that determined for control cells. This difference gradually decreased at higher stirring rates up to 610 rpm when the half-life of the viable cell population was reduced to ∼10 min. Supplementation of cultures with linoleic acid has previously been shown to result in incorporation into all three cellular lipid fractions - polar, non-polar and free fatty acid (Butler et al., 1997). Our explanation for the increased survivability of the cells at high agitation rates in the presence of linoleic acid is that the structural lipid components of the cell including the outer membrane attained a higher unsaturated/saturated ratio which was more robust than that of control cells.
Similar content being viewed by others
References
Al-Rubeai M, Emery AN and Chalder S (1992) The effect of Pluronic F-68 on hybridoma cells in continuous culture. Appl Microbiol Biotechnol 37: 44-45.
Al-Rubeai M, Singh RP, Emery AN and Zhang Z (1995) Cell cycle and cell size dependence of susceptibility to hydrodynamic forces. Biotechnol Bioeng 46: 88-92.
Bailey JM and Dunbar LM (1973) Essential fatty acid requirements of cells in tissue culture: A review. Exp Mol Pathology 18: 142-161.
Bandyopadhyay GK, Imagawa W, Wallace D and Nandi S (1987) Linoleate metabolites enhance the in vitro proliferative response of mouse mammary epithelial cells to epidermal growth factor. J Biol Chem 262: 2750-2756.
Butler M, Huzel N and Barnabé N (1997) Unsaturated fatty acids enhance cell yields and perturb the energy metabolism of an antibody-secreting hybridoma. Biochem J 322: 615-623.
Butler M and Huzel N (1995) The effect of fatty acids on hybridoma cell growth and antibody productivity in serum-free cultures. J Biotechnol 39: 165-173.
Butler M (1986) Serum-free media. In: Thilly WG (ed.) Mammalian Cell Technology (pp. 91-108) Butterworths, Boston.
Calder PC, Bond JA, Bevan SJ, Hunt SV and Newsholme EA (1991) Effect of fatty acids on the proliferation of concanavalin a-stimulated rat lymph node lymphocytes Int J Biochem 23: 579-588.
Calder PC, Yaqoob P, Harvey DJ, Watts A and Newsholme EA (1994) Incorporation of fatty acids by concanavalin A-stimulated lymphocytes and the effect on fatty acid composition and membrane fluidity. Biochem J 300: 509-518.
Cleveland WL, Wood I and Erlanger BF (1983) Routine large-scale production of monoclonal antibodies in a protein-free culture medium. J Immunol Methods 56: 221-234.
Cornwell DG and Morisaki N (1984) Fatty acid paradoxes in the control of cell proliferation: Prostaglandins, lipid peroxides and cooxidation reactions. In: Pryor WA (ed.) Free Radicals in Biology VI (pp. 95-147) Academic Press, New York.
Cuthbert JA and Lipsky PE (1989) Lipoproteins may provide fatty acids necessary for human lymphocyte proliferation by both low density lipoprotein receptor-dependent and independent mechanisms. J Biol Chem 264: 13468-13474.
Doi O, Doi F, Schroeder F, Alberts AW and Vagelos PR (1978) Manipulation of fatty acid composition of membrane phospholipid and its effects on cell growth in mouse LM cells. Biochim Biophys Acta 509: 239-250.
Gillham H and Brindle KM (1996) 31P NMR measurements of the effects of unsaturated fatty acids on cellular phospholipid metabolism. Magnetic Resonance in Medicine 35: 481-488.
Grammatikos SI, Subbaiah PV, Victor TA and Miller WM (1994) Diverse effects of essential (n-6 and n-3) fatty acids on cultured cells. Cytotechnol 15: 31-50.
Handa-Corrigan A, Emery AN, Spier RE (1989) Effect of gas-liquid interfaces on the growth of suspended mammalian cells: mechanisms of cell damage by bubbles. Enzyme Microb Technol 11: 230-235.
Handa-Corrigan A, Weeratunge NP, Braybrook JH, Mackay GA and Chescoe D (1997). In vitro toxicological effects of Pluronic F-68. In: Carrondo MJT, Griffiths B and Moreira JLP (eds) Animal Cell Technology from Vaccines to Genetic Medicine (pp. 121-126) Kluwer Academic Publishers, Dordrecht, The Netherlands.
Jager V, Lehmann J and Friedl P (1988) Serum-free growth medium for the cultivation of a wide spectrum of mammalian cells in stirred bioreactors. Cytotechnol 1: 319-329.
Jordan M, Sucker H, Einsele A, Widmer F and Eppenberger HM (1994) Interactions between animal cells and gas bubbles: the influence of serum and Pluronic F68 on the physical properties of the bubble surface. Biotechnol Bioeng 43: 446-454.
Karsten S, Schafer G and Schauder P (1994) Cytokine production and DNA synthesis by human peripheral lymphocytes in response to palmitic, stearic, oleic and linoleic acid. J Cell Physiol 161: 15-22.
Kawamoto T, Sato JD, Le A, McClure DB and Sato GH (1983) Development of a serum-free medium for growth of NS-1 mouse myeloma cells and its application to the isolation of NS-1 hybridomas. Anal Biochem. 130: 445-453.
Kelly JP and Parker CW (1979) Effects of arachidonic acid and other unsaturated fatty acids on mitogenesis in human lymphocytes. J Immunol 122: 1556-1562.
Kovar J and Franek F (1986) Serum-free medium for hybridoma and parental myeloma cell cultivation. Methods Enzymol 121: 277-292.
Kunas KT and Papoutsakis ET (1990) Damage mechanisms of suspended animal cells in agitated bioreactors with and without bubble entrainment. Biotechnol Bioeng 36: 476-483.
Martens DE, Degooijer CD, Vanderveldendegroot CAM, Beuvery EC and Tramper J (1993) Effect of dilution rate on growth, productivity, cell cycle and size, and shear sensitivity of a hybridoma cell in a continuous culture. Biotechnol Bioeng 41: 429-439.
Michaels JD, Peterson J, McIntire LV and Papoutsakis ET (1991) Protection mechanisms of freely suspended animal cells (CRL8018) from fluid-mechanical injury. Viscometric and bioreactor studies using serum, Pluronic F68 and polyethylene glycol. Biotechnol Bioeng 38: 169-180.
Michaels JD, Nowak JE, Mallik AK, Koczo K, Wasan DT, Papoutsakis ET (1995) Interfacial properties of cell culture media with cell-protecting additives. Biotechnol Bioeng 47: 420-430.
Murakami H (1989) Serum-free media used for the cultivation of hybridomas. In: Mizrahi A (ed.) Monoclonal Antibodies: Production and Applications. (pp. 107-141) Alan R. Liss., New York.
Needleman P, Turk J, Jakschik BA, Morrison AR and Lefkowith JB (1986) Arachidonic acid metabolism. Ann Rev Biochem 55: 69-102.
Papoutsakis ET (1991) Fluid mechanical damage of animal cells in bioreactors. Trends in Biotechnology 9: 427-437.
Petersen JF, McIntire LV and Papoutsakis ET (1988) Shear sensitivity of cultured hybridoma cells (CRL-8081) depends on mode of growth, culture age and metabolic concentration. J Biotechnol 7: 229-246.
Ramirez OT and Mutharasan R (1990) The role of the plasma membrane fluidity on the shear sensitivity of hybridomas grown under hydrodynamic stress. Biotechnol Bioeng 36: 911-920.
Rintoul DA, Sklar LA and Simoni RD (1978) Membrane modification of Chinese hamster ovary cells. J Biol Chem 253: 7447-7452.
Rockwell GA, Sato GH and McClure DB (1980) The growth requirements of SV40 virus transformed Balb/c-3T3 cells in serum-free monolayer culture. J Cell Physiol 103: 323-331.
Rose DP and Connolly JM (1990) Effects of fatty acids and inhibitors of eicosanoid synthesis on the growth of a human breast cancer cell line in culture. Cancer Res 50: 7139-7144.
Rosenthal MD (1987) Fatty acid metabolism of isolated mammalian cells. Prog Lipid Res 26: 87-124.
Schmid G, Zilg H, Eberhard U and Johannsen R (1991) Effect of free fatty acids and phospholipids on growth of and product formation by recombinant baby hamster kidney (rBHK) and Chinese hamster ovary (rCHO) cells in culture. J Biotechnol 17: 155-167.
Spector AA, Mathur SN, Kaduce TL and Hyman BT (1981) Lipid nutrition and metabolism of cultured mammalian cells. Prog Lipid Res 19: 155-186.
Tharakan JP, Lucas A and Chau PC (1986) Hybridoma growth and antibody secretion in serum supplemented and low protein serum-free media. J Immunol Meth 94: 225-235.
Van der Pol L and Tramper J (1998) Shear sensitivity of animal cells from a culture-medium perspective. Trends in Biotechnology 16: 323-328.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Butler, M., Huzel, N., Barnabé, N. et al. Linoleic acid improves the robustness of cells in agitated cultures. Cytotechnology 30, 27–36 (1999). https://doi.org/10.1023/A:1008048126055
Issue Date:
DOI: https://doi.org/10.1023/A:1008048126055