Abstract
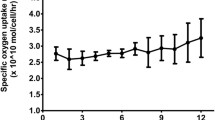
Porcine hepatocytes are used in the hybrid artificial liver support system that we are developing because of their high level of liver functions in vitro and because human hepatocytes can not be used in Japan for ethical reasons. Spherical multicellular aggregates or spheroids have been found to be effective in vitro for long-term maintenance of liver functions. Therefore, we formed spherical multicellular aggregates (spheroids) of primary porcine hepatocytes using a polyurethane foam (PUF) as a culture substratum and analyzed their drug metabolic functions in vitro. Primary porcine hepatocytes inoculated into the pores of a flat PUF plate (25 × 25 × 1 mm), spontaneously formed spheroids within the range of 100 to 150 μm in diameter 24 to 36 h after inoculation. The formed spheroids were attached to the bottom surface of the PUF pores, and their morphology and viability were maintained for more than 12 days. The P-450 activity in the spheroids of porcine hepatocytes was demonstrated by detecting production of monoethylglycinexylidide from lidocaine. In addition, the conjugation enzyme activity was demonstrated by detecting glucuronidation and sulfation of acetaminophen. These activities were maintained for 12 days at a level twice as high as in the monolayer culture. This result shows that the porcine hepatocyte spheroids formed by using PUF can maintain the drug metabolic functions important in a hybrid artificial liver device. Consequently, culturing porcine hepatocyte spheroids using PUF seems to be promising for development of a hybrid artificial liver.
Similar content being viewed by others
References
Bader A, Christians U, Boker K, Sattler M, Sewing K and Pichmayr R (1992) In vitro imitation of the in vivo three-dimensional microenvironment enables primary hepatocytes to maintain stable metabolic functions. Cell Transplantation 1: 162.
Bargetzi MJ, Aoyama T, Gonzalez FJ and Meyer UA (1989) Lidocaine metabolism in human liver microsomes by cytochrome P450IIIA4. Clin Pharmacol Ther 46: 521–527.
Berthiaume F, Moghe PV, Toner M and Yarmush M (1996) Effect of extracellular matrix topology on cell structure, function, and physiological responsiveness: hepatocytes cultured in a sandwich configuration. FASEB J 10: 1471–1484.
Chen SC, Hewitt WR, Watanabe FD, Eguchi S, Kahaku E, Middleton Y, Rozga J and Demetriou AA (1996) Clinical experience with a porcine hepatocyte-based liver support system. Int J Artif Organs 19: 664–669.
Dunn J, Tompkins R and Yarmush M (1992) Hepatocytes in collagen sandwich: evidence for transcriptional and translational regulation. J Cell Biol 116: 1043–1053.
Gerlach JC (1997) Bioreactor for a hybrid liver support. In: Carrondo MJT et al. (eds) Animal Cell Technology (pp. 543–555), Kluwer Academic Publishers, The Netherlands.
Gerlach JC, Brombacher J, Kloppel K, Schnoy N and Neuhays P (1994) Comparison of four methods for mass hepatocyte isolation from pig and human liver. Transplantation 57: 1318–1322.
Guguen GC, Clement B, Baffet G, Beaumont C, Morel CE and Guillouzo A (1983) Maintenance and reversibility of active albumin secretion by adult rat hepatocytes co-cultured with another liver epithelial cell type. Exp Cell Res 143: 47–54.
Hu WS, Friend JR, Wu FJ, Sielaff T, Peshwa MV, Lazar A, Nyberg SL, Remmel RP and Cerra FB (1997) Development of a bioartificial liver employing xenogeneic hepatocytes. Cytotechnology 23: 29–38.
Ijima H, Taniguchi Y, Matsushita T and Funatsu K (1992) Application of three dimensional culture of adult rat hepatocytes in PUF pores for artificial liver support system. In: Murakami H et al. (eds) Animal Cell Technology: Basic and Applied Aspects (pp. 81–86), Kluwer Academic Publishers, The Netherlands.
Kamihira M, Yamada K, Hamamoto R and Iijima S (1997) Spheroid formation of hepatocytes using synthetic polymer. In: Prokop A et al. (eds) Annals of the New York Academy of Siences. vol. 831 (pp. 398–407), The New York Academy of Sciences, New York.
Koide N, Sakaguchi K, Koide Y, Asano K, Kawaguchi M, Matsushota H, Takenami T, Shinji T, Mori M and Tsuji (1990) Formation of multicellular spheroids composed of adult rat hepatocytes in dishes with positively charged surfaces and under other nonadheret enviroments. Exp Cell Res 186: 227–235.
Koide N, Shinji T, Tanabe T, Asano K, Kawaguchi M, Sakaguchi K, Koide Y, Matsushota H, Mori M and Tsuji (1989) Continued high albumin production by multicellular spheroids of adult rat hepatocytes formed in the presence of liver-derived proteoglycans. Biochem Biophys Res Comm 161: 385–391.
Komlot A, Rozga J, Watanabe FD and Demetriou AA (1995) Artificial liver support systems. Biotechnol Bioeng 50: 382–391.
Landry J, Bernier D, Ouellet C, Goyette R and Marceau N (1985) Spheroidal aggregate culture of rat liver cells: Histotypic reorganization, biomatrix deposition, and maintenance of functional activities. J Cell Biol 101: 914–923.
Lazar A, Mann HJ, Remmel RP, Shatford RA, Cerra FB and Hu WS (1995) Extended liver-specific functions of porcine hepatocyte spheroids entrapped in collagen gel. In Vitro Cell Dev Biol 31: 340–346.
Matsushita T, Ijima H, Wada S and Funatsu K (1995) Estimation of the performance of a PUF/spheroid packed-bed type artificial liver by using an extracorporeal circulation with hepatic failure rats. Jpn J Artif Organs 24: 815–820.
Matsushita T, Ijima H and Funatsu K (1992) Development of a hybrid type artificial liver utilizing three dimensional culture of adult hepatocytes. Jpn J Artif Organs 21: 1050–1054.
Matsushita T, Ijima H, Koide N and Funatsu K (1991) High aibumin production by multicellular spheroid of adult rat hepatocytes formed in the pores of polyurethane foam. Appl Microbiol Biotechnol 36: 324–326.
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to poliferation and cytotoxicity assays. J Immunol Methods 65: 55–63.
Nakazawa K, Matsushita T and Funatsu K (1997) Prolonged lidocaine metabolizing activity of primary hepatocytes with spheroid culture using polyurethane foam as a culture substratum. Cytotechnology 24: 235–242.
Naruse K, Sakai Y, Nagashima I, Jiang GX, Suzuki M and Muto T (1996) Comparisons of porcine hepatocyte spheroids and single hepatocytes in the non-woven fabric bioartificial liver module. Int J Artif Organs 19: 605–609.
Nyberg G, Karlen B, Heldlund I, Grundin R and Bahr C (1977) Extraction and metabolism of lidocaine in rat liver. Acta Pharmacol Toxicol 40: 337–346.
Oellerich M, Raude E, Burdelski M, Schulz M, Schmidt FW, Ringe B, Lamesch P, Pichlmayr R, Raith H, Scheruhn M, Wrenger M and Wittekind C (1987) Monoethylglycinexylidide formation kinetics: a novel approach to assessment of liver function. J Clin Chem Clin Biochem 25: 845–853.
Peter M (1978) Paracetamol metabolism and toxicity in isolated hepatocytes from rat and mouse. Biochem Phamacol 27: 2859–2863.
Sakai Y, Naruse K, Nagashima I, Muto T and Suzuki M (1996) Large-scale preparation and function of porcine hepatocyte spheroids. Int J Artif Organs 19: 294–301.
Sakai Y, Furukawa K and Suzuki M (1992) Immobilization and long-term albumin secretion of hepatocyte spheroids rapidly formed by rotational tissue culture methods. Biotech Techniques 6: 527–532.
Seglen PO (1976) Preparation of isolated rat liver cells. In: Prescott DM (ed) Methods Cell Biol. vol. 113 (pp. 29–83), Academic press, New York.
Sielaff TD, Hu MY, Rao S, Groehler K, Olson D, Mann HJ, Remmel RP, Shatford RAD, Amiot B, Hu WS and Cerra FB (1995) A technique for porcine hepatocyte harvest and description of differentiated metabolic functions in static culture. Transplantation 59: 1459–1463.
Studenberg SD and Brouwer KLR (1991) Phenacetin and acetaminophen metabolism in the isolated perfused rat liver. Drug Metab Dispos 19: 423–429.
Takezawa T, Yamazaki M, Mori Y, Yonaha T and Yoshizato K (1992) Morphological and immuno-cytochemical characterization of a hetero-spheroid composed of fibroblasts and hepatocytes. J Cell Science 101: 495–501.
Tong JG, Lagausie PD, Furlan V, Cresteil T, Bernard O and Alvarez F (1992) Long-term culture of adult rat hepatocyte spheroids. Exp Cell Res 200: 326–332.
Rights and permissions
About this article
Cite this article
Nakazawa, K., Mizumoto, H., Kaneko, M. et al. Formation of porcine hepatocyte spherical multicellular aggregates (spheroids) and analysis of drug metabolic functions. Cytotechnology 31, 61–68 (1999). https://doi.org/10.1023/A:1008040726236
Issue Date:
DOI: https://doi.org/10.1023/A:1008040726236