Abstract
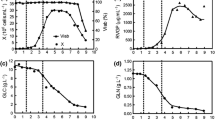
Spodoptera frugiperda (Sf9) insect cells proliferate in a cystine-free medium, with the same growth rate, reaching the same final cell density, as in a cystine-containing medium, provided that the inoculum is taken from a pre-culture sufficiently early, at 47–53 h. With an inoculum from a 103 h culture an extended lag phase accompanied by cell death was observed during the first 50 h of cystine-free culture, even though the culture had been adapted to cystine-free conditions for 10 passages. Cystine-free cultures seeded with a 103 h inoculum had lower growth rates and reached lower final cell densities than corresponding cystine-supplied cultures. Cysteine biosynthesis occurs from methionine via the β-cystathionine pathway. More methionine was consumed by the cells in cystine-free media, and cystathionine was secreted when methionine and cystine were supplied in excess. The data suggest that cysteine biosynthesis is up-regulated in proliferating cells but down-regulated when the cells enter the stationary phase.
In cultures supplied with cystine (10–100 mg 1-1), the specific uptake rate and total consumption of cystine, as well as the uptake of glutamate, glutamine and glucose increased with increasing cystine concentrations. These results are interpreted in view of system x –c , a concentration dependent amino acid transporter. Similarly, the consumption of amino acids transported by system L (ile, leu, val, tyr) was enhanced in cystine-containing cultures, as compared to cystine-free cultures. Uptake of cystine, methionine and system L amino acids ceases abruptly in all cultures, even before growth ceased. The specific growth rate starts to decline early during the growth phase, but this growth behaviour could not be correlated to the depletion of nutrients. We therefore propose that the observed growth pattern is a result of (auto)regulatory events that control both proliferation and metabolism.
Similar content being viewed by others
References
Bannai S (1986) Exchange of cystine and glutamate across plasma membrane of human fibroblasts. J. Biol. Chem. 261: 2256-2263.
Bannai S and Tetsuro I (1988) A novel function of glutamine in cell culture: Utilization of glutamine for the uptake of cystine in human fibroblasts. J. Cell. Physiol. 137: 360-366.
Bédard C, Tom R and Kamen A (1993) Growth, nutrient consumption, and endproduct accumulation in Sf9 and BTIEAA insect cell cultures: Insights into growth limitation and metabolism. Biotechnol. Prog. 9: 615-624.
Bidlingmeyer BA, Cohen SA and Tarvin TL (1985) Rapid analysis of amino acids using precolumn derivatization. J. Chromatogr. 336: 93-104.
Bussolati O, Uggeri J, Belletti SVD and Gazzola GC (1996) The stimulation of Na, K, Cl cotransport and of system A for neutral amino acid transport is an mechanism for cell volume increase during the cell cycle. FASED J. 10: 920-926.
Collarini EJ and Oxender DL (1987) Mechanism of transport of amino acids across membranes. Annu. Rev. Nutr. 7: 75-117.
DelGiudice RA and Hopps HE (1978) Microbiological methods and fluorescent microscopy for the direct demonstration ofmycoplasma infection of cell cultures. In: McGarrity GJ, Murphy DG and Nichols WW (eds.): Mycoplasma infection of cell cultures (pp. 57-69) Plenum Press, New York.
Doverskog M, Ljunggren J, Öhman L and Häggström L (1997) Physiology of cultured animal cells. J. Biotechnol., Submitted.
Drews M, Paalme T and Vilu R (1995) The growth and nutrient utilization of the insect cell line Spodoptera frugiperda Sf9 in batch and continious culture. J. Biotech. 40: 187-198.
Fedorcsak I, HarmsRingdahl M and Ehrenberg L (1977) Prevention of sulfhydryl autoxidation by a polypeptide from red kidney beans, described to be a stimulator of RNA synthesis. Exp. Cell. Res. 108: 331-339.
Ferrance JP, Goel A and Ataai MM (1993) Ultilization of glucose and amino acids in insect cell cultures: Quantifying the metabolic flows within the primary pathways and medium development. Biotechnol. Bioeng. 42: 697-707.
Finkelstein JD and Martin JJ (1986) Methionine metabolism in mammals; Adaptation tomethionine excess. J. Biol. Chem. 261: 1582-1587.
Finkelstein JD, Martin JJ and Harris BJ (1988) Methionine metabolism in mammals; The methionine-sparing effect of cystine. J. Biol. Chem. 263: 11750-11754.
Flavin N (1971) Trans-sulfuration reactions: Introduction. In: Tabor H and Tabor CW(eds.): Methods in Enzymology; Metabolism of amino acids and amines XVIIB. (pp. 416-417) Academic Press, New York.
Flavin M and Slaughter C (1971) γ-Cystathionase (Neurospora). In: Metabolism of amino acids and amines XVIIB. (pp. 433-439) Academic Press, New York.
Guidotti GG and Gazzola GC (1992) Amino acid transporters: Systematic approach and principles of control. In: Kilberg MS and Häussinger D (eds.): Mammalian amino acid transport. (pp. 3-29) Plenum Press, New Uork, NY.
Kilberg MS and Christensen HN (1989) The relation between membrane potential and the transport activity of system A and L in plasma membrane vesicles of the Ehrlich cell. Membrane Biochem. 3: 155-168.
Kioukia N, Nienow AW, Emery AN and Al-Rubeai M(1995) Physiological and environmental factors affecting the growth of insect cells and infection with baculovirus. J. Biotech. 38: 243-251.
Landureau JC and Jillès P (1969) Étude des exigences d'une lignée de cellules d'insectes. Exp. Cell Res. 54: 391-398.
Ljunggren J and Häggström L (1995) Specific growth rate as a parameter for tracing growth limiting substances in animal cell cultures. J. Biotechnol. 42: 163-175.
Makowske M and Christensen CN (1982) Contrasts in transport systems for anionic amino acids in Hepatocytes and a Hepatoma cell line HTC. J. Biol. Chem. 257: 5663-5670.
Mehler AH (1986) Amino Acid Metabolism II: Metabolism of the individual amino acids. In: Devlin TM (ed.): Textbook of Biochemistry. (pp. 453-488) John Wiley and Sons, New York, N.Y.
Mitsuhashi J (1982) Determination of essential amino acids for insect cell lines. In: Invertebrate Cell Culture Applications. (pp. 9-51) Academic Press, Inc. New York.
Neermann J and Wagner R (1996) Comparative analysis of glucose and glutamine metabolism in transformed mammalian cell lines, insect and primary liver cells. J. Cell. Physiol. 166: 152-169.
Neutra R, Levi BZ and Shoham Y (1992) Optimization of protein-production by the baculovirus expression vecctor system in shake flasks. Appl. Microbiol. Biotechnol. 37: 74-78.
Rhiel M and Murhammer DW (1995) The effect of oscillating dissolved oxygen concentrations on the metabolism of a Spodoptera frugiperda IPLBSf21AE clonal isolate. Biotechnol. Bioeng. 47: 640-650.
Shotwell MA, Kilberg MS and Oxender DF (1983) The regulation of neutral amino acid transport in mammalian cells. Biochem. Biophys. Acta 737: 267-284.
Tremblay BG, Meija RN and MacKenzie ER (1992) The NADP-dependent methylenetetrahydrofolate dehydrogenase-methylenetetrahydrofolate cyclohydrolase-formyltetrahydrofolate synthethase is not expressed in Spodoptera frugiperda cells. J. Biol. Chem. 267: 8281-8285.
Wu J, King G, Daugulis AJ, Faulkner P, Bone DH and Goosen MFA (1990) Adaption of insect cells to suspension culture. J. Ferm. Bioeng. 70: 90-93.
Öhman L., Alarcon M, Ljunggren, J. Ramqvist AK and Häggström L (1996) Glutamine is not an essential amino acid for SF9 insect cells. Biotechnol. Lett. 18: 765-770.
Öhman L., Ljunggren J and Häggström L (1995) Induction of a metabolic switch in insect cells by substrate limited fed batch cultures. Appl. Microbiol. Biotechnol. 43: 1006-1013.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Doverskog, M., Han, L. & Häggström, L. Cystine/cysteine metabolism in cultured Sf9 cells: influence of cell physiology on biosynthesis, amino acid uptake and growth. Cytotechnology 26, 91–102 (1998). https://doi.org/10.1023/A:1007963003607
Issue Date:
DOI: https://doi.org/10.1023/A:1007963003607